How to synthesis 2-Bromoethanol
Ethylene bromohydrin (2-Bromoethanol) can be prepared by the reaction between ethylene glycol and hydrobromic acid and phosphorus tribromide, by the direct addition of hypobromous acid to Ethylene, and by the reaction between Ethylene and dilute bromine water. With ethylene oxide now available at a reasonable price, the procedure described has been selected because of the high yields and the convenience of the reaction.
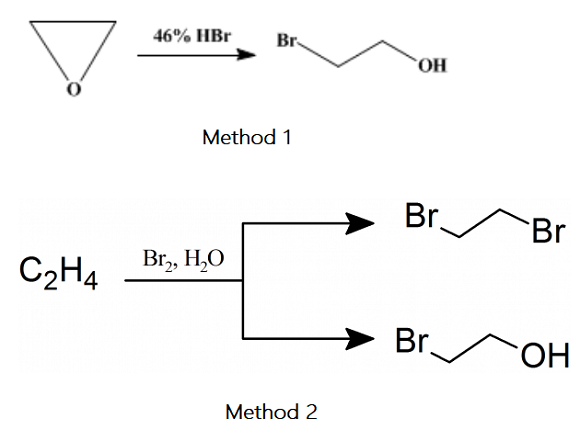
Method 1: Hydrobromic acid and Ethylene oxide
In the 1-l. Three-necked flask A is placed 550 cc. (4.56 mol) of 46 per cent hydrobromic acid (sp. gr. 1.46). Ethylene oxide is added to the acid solution as indicated. Tank E is arranged on a balance so that the amount of ethylene oxide used can be weighed. B is a U-tube containing water to indicate the rate of flow of the gas. D is a glass coil surrounded by ice and salt, which cools the gas nearly to the liquefaction temperature. C is another U-tube containing water, showing whether or not the gas is completely absorbed[1].
The flask A is surrounded by an ice-salt bath, and the stirrer is started. When the temperature of the acid has dropped to 10℃, 132 g (3 mol) of ethylene oxide is added over about two and one-half hours. The stirring is continued for one hour after all the ethylene oxide has been added, and the temperature is maintained below 10 ℃ during the reaction.
After this time, the excess hydrobromic acid is neutralized with excess sodium carbonate, of which about 100 g of anhydrous salt is required. The aqueous solution is then added about 100 g of anhydrous sodium sulfate until some solid does not dissolve. A layer of ethylene bromohydrin separates and is collected in 200 cc. of ether. The ether layer is below the aqueous layer. The solid sodium sulfate is filtered from the solution and washed once or twice with a small amount of ether to remove any mechanically held bromohydrin. The aqueous filtrate is twice extracted with 200-cc. Portions of ether. The combined ether extracts are dried overnight with anhydrous sodium sulfate, filtered and distilled from a steam bath. The remaining bromohydrin is distilled under reduced pressure. After two distillations, the fraction boiling at 55–59℃/22 mm is pure 2-Bromoethanol. The yield is 327–345 g (87–92 per cent of the theoretical amount).
Method 2: Ethylene and bromine
Ethylene gas is washed and passed into an ice-cold solution of 7.2 g of bromine in 500 ml water. After complete absorption of the bromine, a fresh portion of 7.2 g, equal to the first, is added, with frequent and vigorous agitation, until a total weight of 200 g of bromine has reacted. The lower layer of ethylene dibromide, which is formed during the process, is separated, washed with water, and dried over sodium sulfate. Yield, 88 g. After neutralizing with the sodium carbonate and saturating the aqueous layer with sodium chloride, the 2-bromoethanol is extracted by shaking twice with 100 ml of ether, dried over sodium sulfate; the ether is removed by evaporation yielding 85 g of bulk of the residual liquid, which distils between 145-149° C. 54.4% of the bromine is converted into 2-bromoethanol and 37.5% into ethylene dibromide. The remainder is found as hydrobromic acid[2].
References
[1] Organic Syntheses Procedure (orgsyn.org) https://www.orgsyn.org/demo.aspx?prep=CV1P0117.
[2] Preparation of 2-bromoethanol (prepchem.com) https://prepchem.com/synthesis-of-2-bromoethanol/
);You may like
Related articles And Qustion
See also
Lastest Price from 2-Bromoethanol manufacturers

US $0.00/kg2024-08-15
- CAS:
- 540-51-2
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20 tons

US $0.00/KG2023-07-22
- CAS:
- 540-51-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month



