Benzil: Chemical property, Synthesis, and uses
Introduction
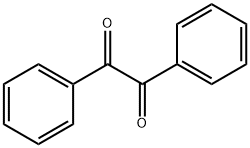
Benzil is one of the eloquent organic compounds consisting of two adjacent carbonyl groups flanked by two phenyl rings. The IUPAC nomenclature consistently designates benzil as 1,2-diphenyl-1,2-dione with the molecular formula C14H10O2. Also known as bis-benzoyl or diphenylglyoxal, benzil belongs to a class of compounds known as stilbenes. These are the organic compounds containing 1,2-diphenyl ethylene moiety containing dicarbonyl system with the general formula RCO COR (R=C6H5)[1].
Chemical property
Benzil is a yellow crystalline solid at room temperature. When dissolved in certain solvents or subjected to certain reactions, it can exhibit a range of colours, including yellow, orange, red and even green. However, its typical colour is yellow. Benzil is a crystalline solid with a mild, pleasant odour. However, the odor of benzil can vary depending on factors such as the purity of the compound, the concentration of the sample, and the individual’s sense of smell[2].
The boiling point of benzil, a diketone compound with the chemical formula C14H10O2, is 344-345°C at a standard atmosphere pressure (101.3kPa). The solubility of benzil varies depending on the solvent used. In general, it is sparingly soluble in water but is a solution in many organic solvents such as ethanol, acetone, and chloroform. According to the Merck Index, the solubility of benzil in water at 20°C is approximately 0.2g/100mL. However, the solubility of benzil in water may vary depending on temperature, pressure, and pH.
With pKa 8.6, benzil is an extremely weak basic (essentially neutral) compound. Benzil has a dipole moment of 3.89 Debye and an ionization potential of 7.7 and 7.5 eV (vertical and adiabatic, respectively) (polarisable continuum model, PCM).
Synthesis
The process is as follows:

Place 2.0 g (0.094 mol) of the crude benzoin and 10 ml of concentrated nitric acid in a 250-ml round-bottomed flask. Heat on a boiling water bath (in the fume cupboard) with occasional shaking until the evolution of oxides of nitrogen has ceased (about 1.5 hours). Pour the reaction mixture into 300-400 ml of cold water in a beaker and stir well until the oil crystallizes completely as a yellow solid. Filter the crude benzil at the pump and wash it thoroughly with water to remove the nitric acid. Recrystallise from ethanol or rectified spirit (about 2.5 ml per gram). The Yield of pure benzil, m.p. 94-96℃, is 1.9 g[3].
Uses
Benzil and its derivatives are widely used in dyes, sensitizers in organic photovoltaics, biomarkers, and fluorescence probes, as well as synthons in synthesising heterocycles with biological and therapeutic applications.[3] In particular, it is frequently used in the free-radical curing of polymer networks, viz., decomposed by ultraviolet radiation that facilitates free-radical species within the material, forming cross-links. Benzil is normally utilized as a photoinitiator in polymer chemistry.[4] Benzil has also demonstrated selective inhibition of human carboxylesterases, which are involved in the hydrolysis of carboxylesters and many clinically used drugs.
References
[1] Dr. Saba Kauser J. Shaikh . “Benzils: A Review on their Synthesis.” Asian Journal of Organic Chemistry 11 2 (2022): Pages 86-131.
[2] Paper10747.pdf (ijarsct.co.in) https://ijarsct.co.in/Paper10747.pdf
[3] IRJET-V7I5717.pdf https://www.irjet.net/archives/V7/i5/IRJET-V7I5717.pdf
);You may like
Related articles And Qustion
Lastest Price from Benzil manufacturers

US $5.00/KG2024-09-19
- CAS:
- 134-81-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200mt/year

US $5.00-2.00/KG2024-09-19
- CAS:
- 134-81-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000kg