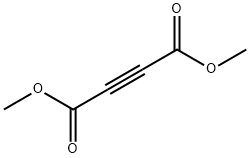
Dimethyl acetylenedicarboxylate - Reaction / Application on Synthetic Work
Dimethyl acetylenedicarboxylate (DMAD) is a useful precursor in organic synthesis[1]. DMAD can undergo Cu(I)-catalyzed radical addition with acetophenones to yield furan derivatives[2]. It is one of the shortest linear links used in the preparation of Covalent Organic Frameworks (COFs) and Metal Organic Frameworks (MOFs) [3].Various hexaarylbenzenes can be built via oxidative cyclodehydrogenation of diaryl alkynes derived from acetylenedicarboxylic acid. It can be used as a spacer while synthesizing molecular chains or dendrimers such as 2,3,8-trifunctionalized indenoquinoxaline dendrons and bisarylacetylene chromophores[4].
DMAD is a di-ester in which the ester groups are conjugated with a C-C triple bond. As such, the molecule is highly electrophilic, and is widely employed as a dienophile in cycloaddition reactions, such as the Diels-Alder reaction. It is also a potent Michael acceptor. This compound exists as a colorless liquid at room temperature. This compound was used in the preparation of nedocromil.
The following example is about its application on the synthesis of coumarin derivatives. [1]
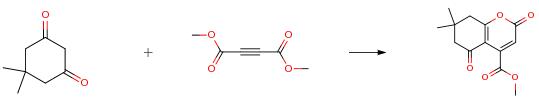
To a magnetically stirred mixture of ethyl acetoacetate (130 mg,1.0mmol) and diethyl acetylenedicarboxylate (204 mg, 1.2mmol) in 1.3 mL dry toluene in a 10 mL round bottom flask, CuO (4 mg,0.05 mmol) as a catalyst was added and stirred for 3 h at 110°C temperature. The progress of the reaction was monitored by TLC. After completion of the reaction, the solvent was evaporated under reduced pressure, and crude product was purified by column chromatography(using 60-120 mesh silica gel) eluting with 10percent ethyl acetate in petroleumether to afford the product (249 mg, 0.83mmol, 83 percent) as a colourless liquid; Rf (20 percent EtOAc/petroleum ether) 0.25
The following example is about its application on the preparation of alkyl rhodanines. [2]
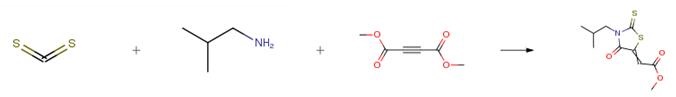
The alkyl amine (2mmol) and carbon disulfide (2mmol) were added to a dispersion of SBA-15 (20mg) in HFIP (4mL), and the mixture was stirred at room temperature for 30 Sec, and then 2mmol of DMAD was added to the mixture during 30 s. After completion of the reaction (TLC), the SBA-15 and TFIP was drawn out and the crude rhodanines were washed with cold diethyl ether. All isolated products gave satisfactory spectral and physical data.
The following example is about its application on the synthesis of synthesis of rhodanines. [3]
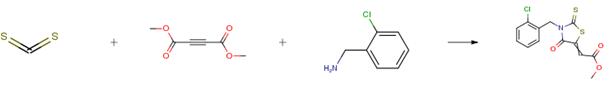
1 mmol of benzylamine and 1 mmol of dimethyl acetylenedicarboxylate (DMAD) were mixed together in the presence of water dispersed Fe3O4(at)KCAR (0.01 g/2 mL) and then, water dissolved CS2 (1 mmol/1 mL) was added to the reaction mixture during 5 min. After 5 min, the color of the reaction mixture turned to orange and the reaction completion was monitored by TLC. Finally, an extra amount of water and then dichloromethane were added to the solution to stop the reaction and extract the product. Thus, the product was transferred to dichloromethane and the catalyst was separated from the mixture with a magnet. Then the dichloromethane was evaporated and the residue was purified by column chromatography if needed. The catalyst was washed with ethanol/ether and used for the next cycles (in the case of recyclability test).
The following example is about its application on the synthesis of functionalized dihydropyridines [4]
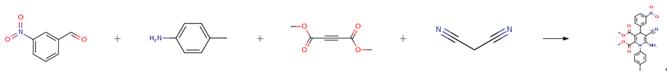
A solution of 140 mg 4-chlorobenzaldehyde (1.0 mmol), 66 mg malononitrile (1.0 mmol), and meglumine (0.1 mmol) were stirred in 1 cm3 of aqueous ethanol solution at room temperature. Then, a solution of 107 mg 4-methylaniline(1.0 mmol) and 142 mg dimethyl acetylenedicarboxylate(1.0 mmol) in 2 cm3 aqueous ethanol solution was added. The resulting mixture was stirred until the reaction was completed as indicated by thin-layer chromatography (TLC). The resulting precipitates were collected by filtration and washed with ethanol. The crude product was purified by crystallization from ethanol to give pure product.
References
1.Kayal U, Karmakar R, Banerjee D, Maiti G. Copper oxide catalyzed domino process for the synthesis of substituted 2H-pyran-2-ones and polyhydroxy coumarin derivatives[J]. Tetrahedron, 2014, 70(39):7016-7021.
2.Rostamnia S, Doustkhah E, Nuri A. Hexafluoroisopropanol dispersed into the nanoporous SBA-15 (HFIP/SBA-15) as a rapid, metal-free, highly reusable and suitable combined catalyst for domino cyclization process in chemoselective preparation of alkyl rhodanines[J]. Journal of Fluorine Chemistry, 2013, 153:1-6.
3.Rostamnia S, Zeynizadeh B, Doustkhah E, Baghban A, Aghbash KO. The use of κ-carrageenan/Fe3O4 nanocomposite as a nanomagnetic catalyst for clean synthesis of rhodanines[J]. Catalysis Communications, 2015, 68:77-83.
4.Chen HS, Guo RY. Meglumine: An efficient, biodegradable, and recyclable green catalyst for one-pot synthesis of functionalized dihydropyridines[J]. Monatshefte fur Chemie, 2015, 146(8):1355-1362.
You may like
Related articles And Qustion
See also
Lastest Price from Dimethyl acetylenedicarboxylate manufacturers

US $10.00/KG2024-05-28
- CAS:
- 762-42-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $10.00/KG2024-05-28
- CAS:
- 762-42-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt