-
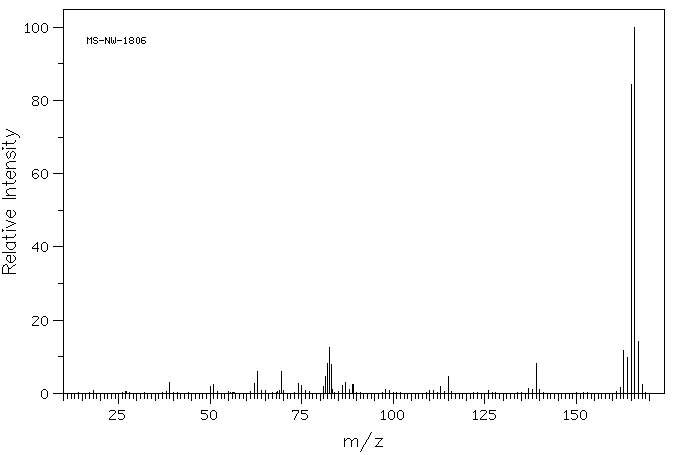
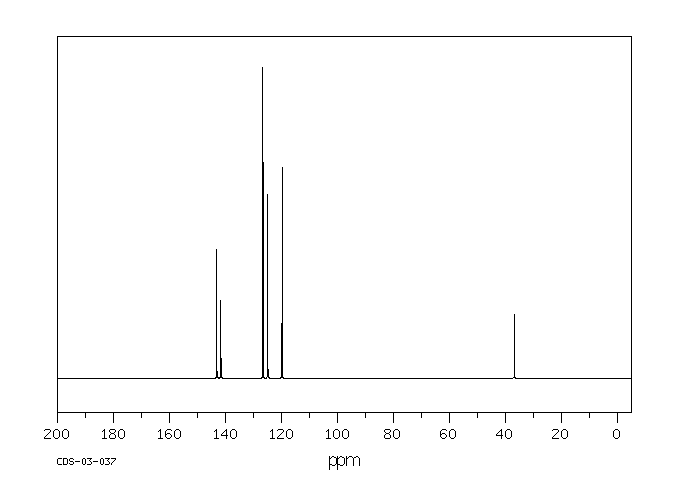
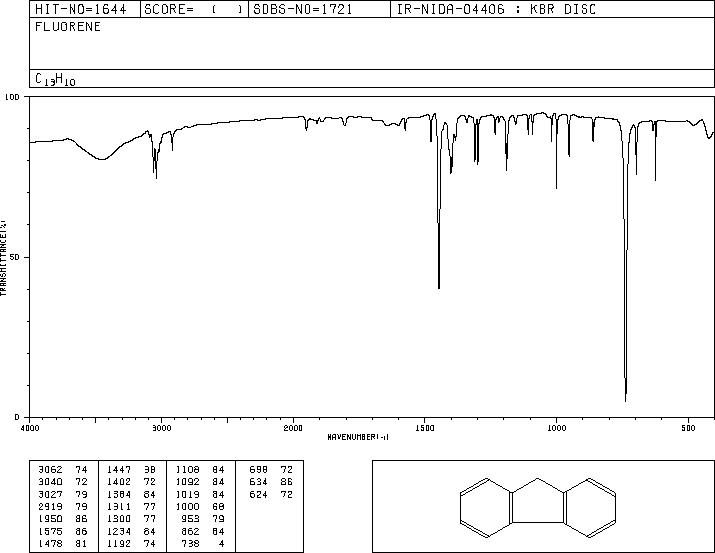
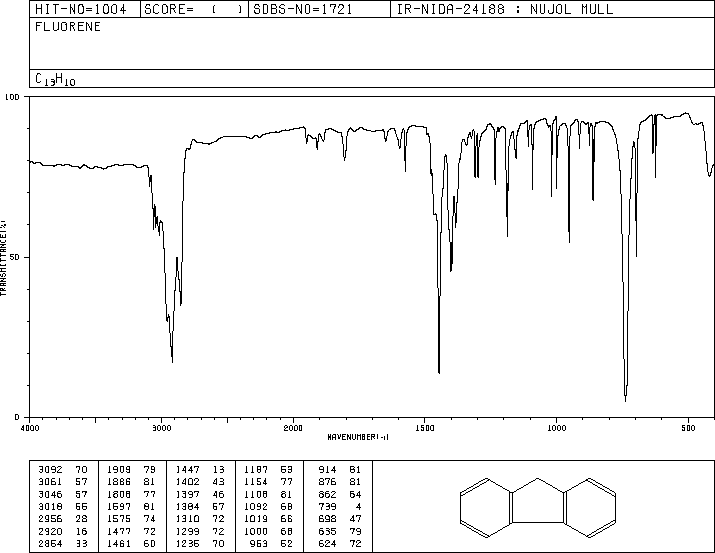
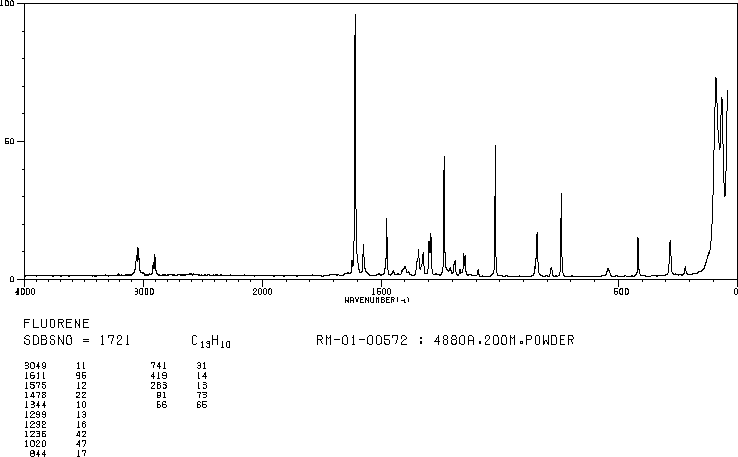
MS-NW-1806 fluorene C13H10 (Mass of molecular ion: 166)
Source Temperature: 240 °C Sample Temperature: 180 °C Reservoir, 75 eV
39.0 3.0 50.0 2.0 51.0 2.3 62.0 2.7 63.0 6.0 69.5 6.0 74.0 2.7 75.0 2.1 81.0 2.0 81.5 4.7 82.0 8.2 82.5 12.4 83.0 8.0 83.5 1.0 86.0 2.1 87.0 2.9 88.0 1.0 89.0 2.5 98.0 1.1 113.0 2.0 115.0 4.6 137.0 1.2 138.0 1.1 139.0 8.1 140.0 1.0 162.0 1.5 163.0 11.8 164.0 9.9 165.0 84.5 166.0 100.0 167.0 14.1 168.0 2.5
parameter in cyclohexane
parameter in CDCl3
400 MHz in CDCl3
parameter in CCl4
parameter in acetone-d6
-
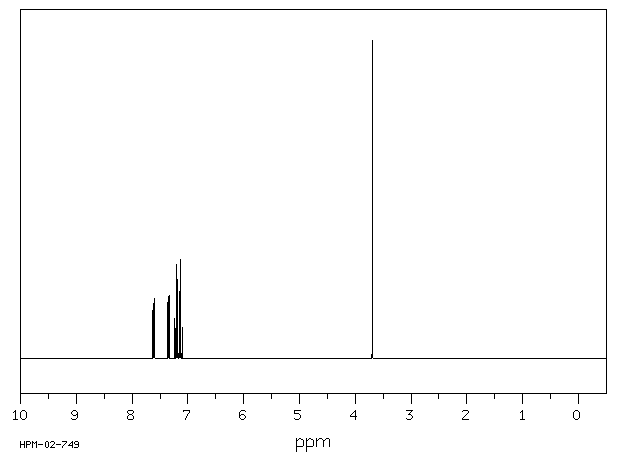
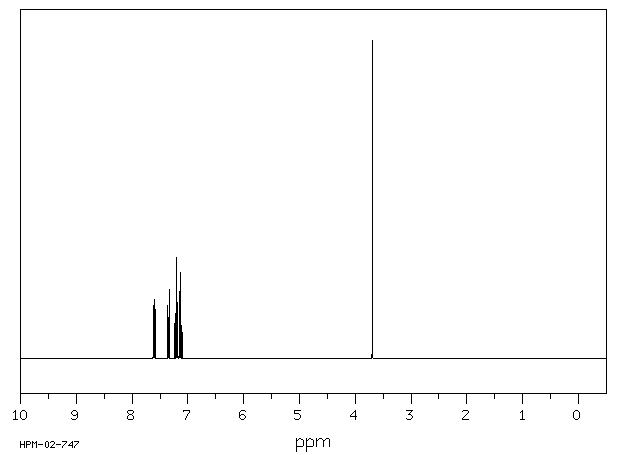
1H NMR 300 MHz C13 H10 0.602 M in cyclohexane fluorene 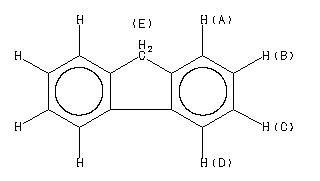
Parameter ppm Hz
D(A) 7.352 D(B) 7.133 D(C) 7.207 D(D) 7.615 J(A,B) 7.76 J(A,C) 1.07 J(A,D) 0.86 J(B,C) 7.62 J(B,D) 1.12 J(C,D) 7.64 D(E) 3.70 DOURIS,J. & MATHIEU,A. BULL.SOC.CHIM.FR. 1971, 3365
Hz ppm Int.2289.39 7.631 149 2288.51 7.628 192 2288.14 7.627 194 2287.41 7.625 161 2282.01 7.607 163 2280.88 7.603 243 2279.85 7.599 172 2210.70 7.369 139 2209.57 7.365 196 2208.50 7.362 140 2203.07 7.344 181 2201.97 7.340 236 2201.02 7.337 179 2170.79 7.236 100 2169.58 7.232 110 2163.24 7.211 258 2161.99 7.207 254 2155.79 7.186 192 2154.47 7.182 164 2147.24 7.157 203 2145.96 7.153 211 2139.69 7.132 255 2138.40 7.128 241 2132.06 7.107 90 2130.85 7.103 83 1109.98 3.700 1000
-
1H NMR 300 MHz C13 H10 0.991 M in CDCl3 fluorene 
Parameter ppm Hz
D(A) 7.447 D(B) 7.221 D(C) 7.294 D(D) 7.705 J(A,B) 7.77 J(A,C) 1.08 J(A,D) 0.76 J(B,C) 7.61 J(B,D) 1.16 J(C,D) 7.61 D(E) 3.79 DOURIS,J. & MATHIEU,A. BULL.SOC.CHIM.FR. 1971, 3365
Hz ppm Int.2316.34 7.721 152 2315.61 7.719 182 2315.02 7.717 187 2314.40 7.715 166 2309.00 7.697 167 2308.12 7.694 210 2307.68 7.692 212 2306.91 7.690 177 2239.09 7.464 148 2238.35 7.461 182 2237.95 7.460 183 2237.03 7.457 143 2231.50 7.438 189 2230.87 7.436 220 2230.32 7.434 224 2229.59 7.432 186 2196.87 7.323 99 2195.66 7.319 109 2189.31 7.298 254 2188.10 7.294 256 2181.90 7.273 193 2180.58 7.269 165 2173.68 7.246 202 2172.33 7.241 210 2166.09 7.220 255 2164.81 7.216 241 2158.50 7.195 90 2157.25 7.191 82 1137.01 3.790 1000
-
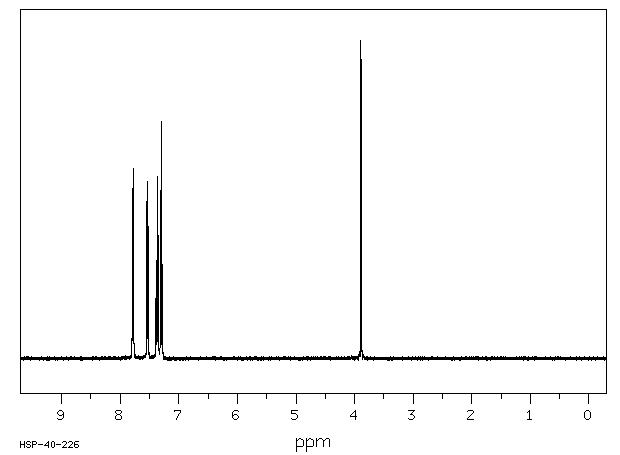
1H NMR 399.65 MHz C13 H10 1.60 mg : 0.5 ml CDCl3 fluorene 
Assign. Shift(ppm)
A 7.783 B 7.536 C 7.367 D 7.297 E 3.889
Hz ppm Int.3114.62 7.794 535 3111.08 7.785 63 3107.06 7.775 597 3016.48 7.548 412 3015.63 7.546 493 3014.53 7.543 361 3012.21 7.538 66 3009.03 7.530 480 3008.30 7.528 558 3007.08 7.525 408 3004.52 7.518 49 2952.76 7.389 250 2952.27 7.388 228 2951.66 7.386 222 2949.34 7.380 57 2948.12 7.377 55 2945.31 7.370 574 2944.82 7.369 517 2944.21 7.367 499 2941.53 7.361 59 2940.80 7.359 59 2937.87 7.352 386 2937.38 7.350 335 2936.65 7.349 311 2923.10 7.315 528 2921.88 7.312 520 2919.92 7.307 51 2919.31 7.305 52 2918.46 7.303 59 2917.85 7.302 71 2915.77 7.296 745 2914.55 7.293 690 2911.87 7.287 50 2910.52 7.283 48 2908.33 7.278 296 2907.10 7.275 262 1554.44 3.890 1000
-
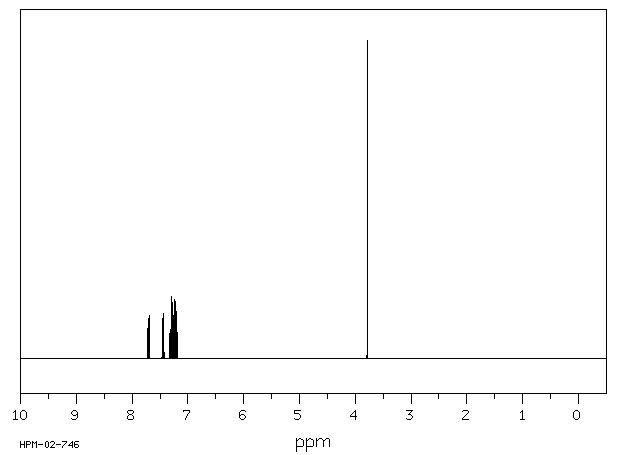
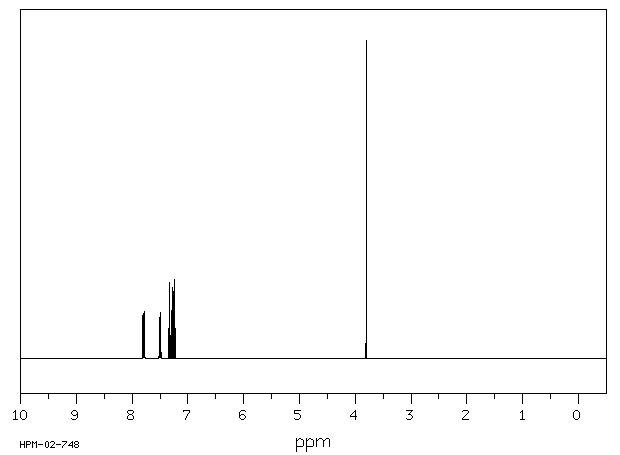
1H NMR 300 MHz C13 H10 0.980 M in CCl4 fluorene 
Parameter ppm Hz
D(A) 7.349 D(B) 7.135 D(C) 7.208 D(D) 7.605 J(A,B) 7.71 J(A,C) 1.02 J(A,D) 0.77 J(B,C) 7.53 J(B,D) 1.13 J(C,D) 7.67 D(E) 3.70 DOURIS,J. & MATHIEU,A. BULL.SOC.CHIM.FR. 1971, 3365
Hz ppm Int.2286.38 7.621 152 2285.61 7.619 186 2285.09 7.617 190 2284.47 7.615 166 2278.97 7.597 166 2277.72 7.592 224 2276.91 7.590 179 2209.68 7.366 144 2208.65 7.362 199 2207.66 7.359 143 2202.12 7.340 191 2201.46 7.338 226 2201.02 7.337 231 2200.25 7.334 187 2171.01 7.237 100 2169.84 7.233 111 2163.53 7.212 266 2162.32 7.208 260 2156.08 7.187 193 2154.80 7.183 165 2147.79 7.159 203 2146.47 7.155 211 2140.27 7.134 255 2138.99 7.130 243 2132.75 7.109 89 2131.51 7.105 82 1109.98 3.700 1000
-
1H NMR 300 MHz C13 H10 1.005 M in acetone-d6 fluorene 
Parameter ppm Hz
D(A) 7.501 D(B) 7.254 D(C) 7.324 D(D) 7.792 J(A,B) 7.79 J(A,C) 1.09 J(A,D) 0.81 J(B,C) 7.47 J(B,D) 1.17 J(C,D) 7.53 D(E) 3.81 DOURIS,J. & MATHIEU,A. BULL.SOC.CHIM.FR. 1971, 3365
Hz ppm Int.2342.42 7.808 150 2341.60 7.805 184 2341.08 7.804 188 2340.41 7.801 163 2335.19 7.784 162 2333.88 7.780 217 2333.02 7.777 172 2255.31 7.518 146 2254.19 7.514 186 2253.18 7.511 142 2247.70 7.492 187 2247.07 7.490 216 2246.54 7.488 218 2245.69 7.486 179 2205.78 7.353 97 2204.55 7.348 108 2198.36 7.328 255 2197.09 7.324 257 2191.05 7.303 195 2189.71 7.299 168 2183.56 7.279 201 2182.18 7.274 210 2175.99 7.253 242 2174.64 7.249 242 2168.45 7.228 90 2167.19 7.224 82 1142.98 3.810 1000
更多供应商