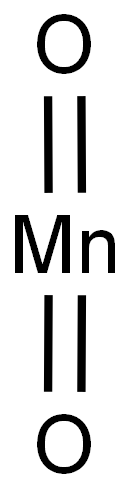Hazard
Oxidizing agent, may ignite organic materials.
Potential Exposure
Manganese dioxide is used as depolarizer for dry cell batteries, for production of manganese metal; as an oxidizing agent; laboratory reagent;
and in making pyrotechnics and matches; in dry cell
batteries.
First aid
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts
the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from
exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has
stopped and CPR if heart action has stopped. Transfer
promptly to a medical facility. When this chemical has
been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
Shipping
UN1479 Oxidizing solid, n.o.s., Hazard Class:
5.1; Labels: 5.1-Oxidizer, Technical Name Required.
UN3137 (powder) Oxidizing solid, flammable, Hazard
Class: 5.1; Labels: 5.1-Oxidizer, 4.1 Flammable solid,
Technical Name Required.
Incompatibilities
A powerful oxidizer. Incompatible with
strong acids; reducing agents; combustible materials (such as
fuel and clothing; organic materials. Mixtures with calcium
hydride is a heat- and friction-sensitive explosive. Vigorous
reaction with hydrogen sulfide, diboron tetrafluoride; calcium hydride; chlorine trifluoride; hydrogen peroxide; hydroxyaluminum chloride; anilinium perchlorate. Decomposes
when heated above 553�C producing manganese(III)oxide
and oxygen, which increases fire hazard. Reacts violently
with aluminum (thermite reaction), potassium azide; rubidium acetylide; in the presence of hea
Waste Disposal
Generators of waste (equal to
or greater than 100 kg/mo) containing this contaminant,
EPA hazardous waste number N450, must conform to
USEPA regulations for storage, transportation, treatment,
and disposal of waste. Dispose of waste material as
hazardous waste using a licensed disposal contractor to an
approved landfill. Dispose of contents and container to
an approved waste disposal plant. Containers must be
disposed of properly by following package label directions
or by contacting your local or federal environmental
control agency, or by contacting your regional EPA
office. All federal, state, and local environmental regulations must be observed. Do not discharge into drains
or sewers
General Description
Manganese(IV) oxide (MnO2) is an eco-friendly chemical having a high theoretical specific capacitance.
Flammability and Explosibility
Nonflammable
Industrial uses
Manganese dioxide (MnO2) is soluble in water and HNO3 and soluble in HCl. It occurs in nature as the blue-black mineral pyrolusite. In glass, manganese dioxide is used as a colorant and decolorizer.
The major use of manganese oxides is an ore of manganese for the manufacturing of steel; manganese serves to increase the hardness and decrease the brittleness of steel. Another important use of manganese oxides is as the cathode material of common zinc/carbon and alkaline batteries (such as flashlight batteries).
storage
Color Code—Yellow: Reactive Hazard (strong oxidizer); Store in a location separate from other materials, especially flammables and combustibles. Prior to working with this chemical you should be trained on its proper handling and storage. Manganese dioxide must be stored to avoid contact with heat and flammable materials and oxidizers (such as perchlorates, peroxides, permanganates, chlorates, and nitrates), since violent reactions occur. See also incompatibilities above. See OSHA Standard 1910.104 and NFPA 43A Code for the Storage of Liquid and Solid Oxidizers for detailed handling and storage regulations.