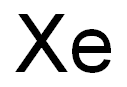Xenon
Feb 21,2022
Xenon is a dense, colourless gas found in the Earth's atmosphere in trace
amounts. Cullen and Gross first reported the anaesthetic properties of xenon
in humans in 1951.
Xenon offers many advantages over nitrous oxide, for which it could
theoretically become a suitable replacement. Unfortunately it is difficult to
manufacture, which makes it extremely expensive, precluding its use in
mainstream anaesthesia. However, anaesthetic machines with the ability to
deliver xenon are likely to become available thanks to a mechanism by which
xenon can be recovered and recycled, making it far more economical.
Xenon, in common with nitrous oxide and ketamine, acts by non-competitive inhibition of NMDA receptors in the CNS.Physical properties
Xenon has a blood/gas partition coefficient of 0.14–0.2, which is lower than that of nitrous oxide (0.47) and therefore provides rapid induction of and recovery from anaesthesia. Xenon is more potent than nitrous oxide, with a MAC of 70%. It does not undergo biotransformation, and it is harmless to the ozone layer.
Systemic effects
Studies so far have found no cardiorespiratory adverse effects or reduction in local organ perfusion; in fact, to date it has demonstrated both cardio- and neuroprotective properties through a variety of mechanisms. It is not irritating to the respiratory tract. Xenon is a competitive inhibitor of the 5-HT3 receptor and is therefore thought to reduce the incidence of PONV.
);