What is Triethylamine trihydrofluoride?
Feb 18,2020
The t-butyldimethylsilyl (TBDMS) group is the most widely used 2'-OH protection in synthesis of oligoribonucleotides. The TBDMS-group became much used particularly after it was combined with phosphoroamidite methodology [1]and was also the choice when H-phosphonate based RNA-synthesis was introduced [2]. Removal of TBDMS-protection is most commonly done with 1 M tetrabutylammonium fluoride (TBAF) in tetrahydrofuran (THF). These conditions has proven efficient, but it has been reported that deprotection may be incomplete for longer RNA's such as a t-RNA [3].
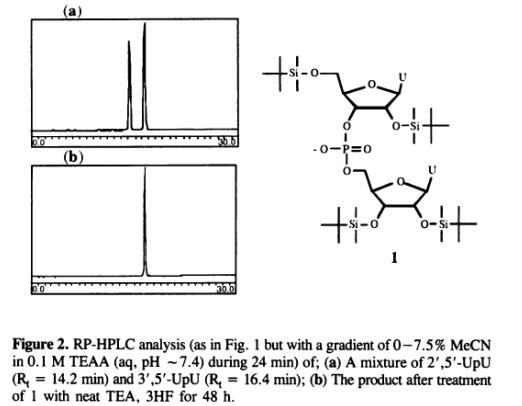
Neat triethylamine trihydrofluoride was reported to be a more efficient desilylating agent than TBAF and successful in deprotecting a synthetic t-RNA [3]. When TEA·3HF was used directly from the bottle, all-2'-O-TBDMS-(UP)20U was deprotected faster than with the standard TBAF solution. TEA·3HF removes the silyl groups within l h . Importantly the rate of desilylation with this reagent is also quite insensitive to the presence of moisture. A reagent with 1 M higher water concentration (from added extra water, 1.8% - total water content 2.2% w/w) still caused complete deprotection after 1 h and this was true even with 3 M higher water content (total water content -5.8% w/w). The TEA·3HF reagent also does not seem to take up moisture particularly fast since bottles that were opened and reopened over more than a month only contained about 0.3-0.35 M water (-0.6-0.7% w/w, typical water content when newly opened was around 0.4%w/w). Deprotection when using TEA·3HF is clearly not particularly sensitive to moisture and this should give a high procedure and faster deprotection. Longer oligomers may require longer deprotection time than for the 21-mers in this study and was also reported to be between 2 and 4 h for a 43-mer (3). It have been turned to using this reagent rather than TBAF and to make certain that deprotection will be complete we are using longer deprotection times than should be necessary (4-8 h and often overnight for convenience) since this does not seem to cause any damage. Thus, (Up)20U remains unchanged when kept in the reagent overnight.
Using 1 M TBAF in THF is an established method for removal of TBDMS-groups in synthesis of RNA-fragments. The reagent does however have the disadvantage of being quite sensitive to moisture. The water content can be kept down by lyophilizing the reagent before use (or drying the solution with molecular sieves) but the TBAF-reagent is still not as efficient as TEA·3HF and also requires more tedious workup (as well as prepreparation). It has been confirmed that TEA·3HF is more efficient than TBAF for TBDMS-deprotection of oligoribonucleotides. The TEA, 3HF reagent has the additional advantage of being quite insensitive to moisture which should ensure a high reliability when using the reagent straight from the bottle. In addition TEA·3HF is quite safe with respect to migration of phosphodiester linkages since this event can not be detected in the product, 3',5'-UpU, even after 48 h treatment of 1 with TEA·3HF. [4]
References
1.Usman,N, Pon,RT, Ogilvie KK. Removal of t-butyldimethylsilyl protection in RNA-synthesis. Triethylamine trihy[J]. Tetrahedron Lett., 1985,26:4567-4570.
2.Garegg PJ, Lindh I, Regberg T, Stawinski J, Stromberg R, Henrichson C. Nucleoside H-phosphonates. IV. Automated solid phase synthesis of oligoribonucleotides by the hydrogenphosphonate approach[J]. Tetrahedron Lett. 1986, 27:4055-4058.
3.Gasparutto D, Livache T, Bazin H, Duplaa A-M, Guy A, Khorlin A, Molko D, Roget A, Teoule R. Chemical synthesis of a biologically active natural tRNA with its minor bases[J]. Nucleic Acids Res. 1992, 20:5159-5166
Erik W, Roger S. Removal of t-butyldimethylsilyl protection in RNA-synthesis. Triethylamine trihydrofluoride (TEA, 3HF) is a more reliable alternative to tetrabutylammonium fluoride (TBAF). Nucleic Acids Research, 1994, 22(12): 2430-2431
);
You may like
What you need to know about ceramides?
Apr 17, 2024
A formaldehyde releasing agent: Quaternium-15
Jan 12, 2024
Oleamide: Physiological effects
Jul 18, 2023
Related articles And Qustion
Lastest Price from Triethylamine trihydrofluoride manufacturers
Triethylamine trihydrofluoride

US $0.00/kg2024-09-12
- CAS:
- 73602-61-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 50000KG/month
Triethylamine trihydrofluoride
US $0.10/KG2024-07-15
- CAS:
- 73602-61-6
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 1000 tons