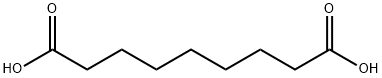
What is Azelaic Acid?
Azelaic acid, also known as azalea acid, is a white to slightly yellow powder. Azelaic acid is a medium-long chain dibasic acid[1]. In recent years, with the rapid development of the organic synthetic chemical industry, the demand for medium and long chain dibasic acids is increasing[2]. The medium and long chain dibasic acids and their derivatives have a wide range of industrial applications and a broad product market[3].
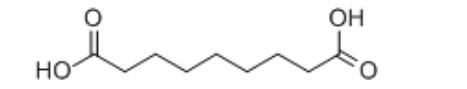
1 Oxidative cleavage reaction of unsaturated fatty acids
Using unsaturated fatty acids or their derivatives as raw materials, anoic acid and azelaic acid are prepared by oxidative cracking. This is the earliest industrialized method, and it is also a method that is widely valued[4]. Commonly used unsaturated fatty acids are oleic acid, linoleic acid, castor oil, etc., of which oleic acid is the most commonly used raw material. There are also many types of oxidants used in the reaction, and commonly used are ozone, nitric acid, hydrogen peroxide, hypochlorite and the like. Emery Industries in the United States first produced Nonanoic acid and azelaic acid from oleic acid using ozone as an oxidant in industrial production[5].
2 Oxidation of organic substances such as aldol
Azelaic acid can be obtained from the corresponding glycol or dialdehyde by oxidation. Kuraray Co. Ltd uses azelaic acid as raw material, acetic acid as solvent, and oxidation of oxygen in the presence of iron acetate catalyst to produce azelaic acid[6]. Saito et al. Used metal platinum as the catalyst, water as the solvent, and oxidized with air to produce azelaic acid with nonanediol. The conversion rate was greater than 98% and the yield was 82%. When acetic acid was used as the solvent and copper acetate was used as the catalyst, the conversion rate was 93% and the yield was 80%.
3 biochemical reactions
In the synthesis of binary acids, it is of great significance to use microbial fermentation and oxidation to convert n-alkanes to binary acids. This method has sufficient raw material sources, low cost, high product purity, and generally does not produce pollution, which is a more promising production method[7].
References
[1] Shemer A, Weiss G, Amichai B, et al. Azelaic acid (20%) cream in the treatment of acne vulgaris[J]. 2010, 16(2):178-179.
[2] Luis M. Bali?a, Klaus Graupe. The Treatment of Melasma 20% Azelaic Acid versus 4% Hydroquinone Cream[J]. International Journal of Dermatology, 30(12):893-895.
[3] E. Esposito, E. Menegatti, R. Cortesi. Ethosomes and liposomes as topical vehicles for azelaic acid: a preformulation study[J]. Journal of Cosmetic Science, 2004, 26(5):270-271.
[4] Sarkar, Rashmi, Bhalla, Mala, Kanwar, Amrinder J. A Comparative Study of 20% Azelaic Acid Cream Monotherapy versus a Sequential Therapy in the Treatment of Melasma in Dark-Skinned Patients[J]. Dermatology, 205(3):249-254.
[5] Liming Yang, Madhumita B. Ray, Liya E. Yu. Photooxidation of dicarboxylic acids—Part I: Effects of inorganic ions on degradation of azelaic acid[J]. Atmospheric Environment, 42(5):856-867.
);Related articles And Qustion
Lastest Price from Azelaic acid manufacturers

US $0.00-0.00/kg2024-09-19
- CAS:
- 123-99-9
- Min. Order:
- 0.10000000149011612kg
- Purity:
- 99%
- Supply Ability:
- 2000kgs

US $100.00-75.00/kg2024-09-19
- CAS:
- 123-99-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000