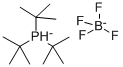Tri-tert-butylphosphine tetrafluoroborate: Overview and Role in Catalytic Reaction
General Description
Tri-tert-butylphosphine tetrafluoroborate, known for its stability and distinct molecular structure, serves as a crucial catalyst in organic transformations. Its applications include catalyzing selective α-arylation reactions and facilitating Sonogashira couplings in aqueous environments. The compound's stability, despite its reactivity, necessitates careful handling and storage under inert conditions. By leveraging its steric effects, Tri-tert-butylphosphine tetrafluoroborate enables precise control over reaction pathways, vital for synthesizing complex molecules like pharmaceuticals and polymers. Overall, its versatility and efficiency in promoting diverse chemical reactions highlight its significant role in advancing modern synthetic chemistry, offering solutions to challenging synthetic problems with high selectivity and yield.

Figure 1. Tri-tert-butylphosphine tetrafluoroborate
Overview
Chemical Structure and Stability
Tri-tert-butylphosphine tetrafluoroborate is a chemical compound characterized by its distinct molecular structure, consisting of a phosphorus atom bonded to three tert-butyl groups and associated with a tetrafluoroborate anion. This configuration imparts significant stability to the molecule, making Tri-tert-butylphosphine tetrafluoroborate resistant to decomposition under normal handling and storage conditions. The tetrafluoroborate anion stabilizes the phosphine, preventing oxidation and degradation that are common with simpler phosphine compounds. This stability is critical for its applications in various chemical reactions where a stable, non-oxidizing environment is crucial. 1
Applications
Tri-tert-butylphosphine tetrafluoroborate is primarily utilized as a ligand in catalytic processes, particularly in the field of organic synthesis. Its bulky tert-butyl groups help create a steric effect that can significantly influence the course of a chemical reaction. By adjusting reaction selectivity and rates, Tri-tert-butylphosphine tetrafluoroborate enables the development of more efficient catalytic systems. This has implications for the synthesis of complex molecules, including pharmaceuticals and polymers, where precise control over reaction pathways is necessary to achieve desired outcomes.
Handling and Safety Considerations
Handling Tri-tert-butylphosphine tetrafluoroborate requires careful consideration of safety protocols due to its reactive nature, despite its inherent stability. Proper storage conditions involve keeping the compound in an inert atmosphere, typically under nitrogen or argon, to prevent any potential degradation through exposure to air. Additionally, safety measures must include wearing appropriate protective gear and ensuring that all containers and handling equipment are suitable for use with reactive phosphine compounds. These precautions help mitigate the risks associated with handling Tri-tert-butylphosphine tetrafluoroborate, preserving its integrity for industrial and laboratory use. 1
Role in Catalytic Reaction
Tri-tert-butylphosphine tetrafluoroborate) has emerged as a versatile catalyst in various organic transformations due to its unique properties and effectiveness in catalyzing complex reactions. This compound plays a pivotal role in mediating selective α-arylation reactions of α,β-unsaturated imides under visible light photoredox conditions, showcasing its broad applicability and efficiency in synthetic organic chemistry.
Selective α-Arylation of α,β-Unsaturated Imides
Tri-tert-butylphosphine tetrafluoroborate facilitates the α-arylation of α,β-unsaturated imides via a visible light photoredox catalytic cycle. This transformation involves the generation of aminium radicals from diarylalkylamines under the influence of a photoredox catalyst. By effectively quenching the emission of the catalyst through experimental methods, researchers have elucidated a plausible reaction pathway. The outcome of this process is the synthesis of valuable compounds such as 3,5-bis(1,1-dimethylethyl)-N-methyl-N-phenylbenzenamine in excellent yields, exemplifying the efficiency of Tri-tert-butylphosphine tetrafluoroborate in enabling precise and high-yielding carbon-carbon bond formations. 2
Application in Sonogashira Coupling with Aqueous Ammonia
Another notable application of Tri-tert-butylphosphine tetrafluoroborate lies in its role in the Sonogashira coupling reaction involving terminal alkynes and aryl bromides in the presence of aqueous ammonia. The catalyst participates in forming an in-situ palladium complex with bulky alkylphosphines, such as Tri-tert-butylphosphine tetrafluoroborate, facilitating the coupling under mild conditions at room temperature. This method allows for the one-pot synthesis of benzofuran and indole derivatives through subsequent intramolecular cyclization, showcasing the catalyst's ability to mediate complex transformations efficiently. 3
Tri-tert-butylphosphine tetrafluoroborate stands out as a versatile and effective catalyst in organic synthesis, particularly in the selective α-arylation of α,β-unsaturated imides under visible light photoredox conditions and in the Sonogashira coupling with aqueous ammonia. Its application underscores its importance in modern synthetic chemistry, enabling the synthesis of diverse and complex organic molecules with high efficiency and selectivity. As research continues to explore its potential, Tri-tert-butylphosphine tetrafluoroborate is poised to further advance the field of catalysis, offering innovative solutions to challenging synthetic problems.
Reference
1. National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 2734635. Tri-tert-butylphosphonium tetrafluoroborate.
2. Ando Y, Kamatsuka T, Shinokubo H, Miyake Y. Selective α-arylation of α,β-unsaturated imides mediated by a visible light photoredox catalyst. Chemical Communications. 2017; 53(65): 9136-9138.
3. Fukuoka S, Naito T, Sekiguchi H, Somete T, Mori A. Effect of the use of bulky alkylphosphines in the Sonogashira coupling with aqueous ammonia. Heterocycles. 2008; 76(1): 819-826.
);Related articles And Qustion
Lastest Price from Tri-tert-butylphosphine tetrafluoroborate manufacturers
US $12.00-260.00/g2024-09-12
- CAS:
- 131274-22-1
- Min. Order:
- 25g
- Purity:
- 0.98
- Supply Ability:
- 25kg

US $0.00-0.00/KG2024-05-28
- CAS:
- 131274-22-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt