The structure of Vanadium carbide
Vanadium carbides display a unique combination of thermal and mechanical properties such as high hardness, high melting point, high-temperature strength, corrosion and wear resistance, and very efficient electrical and thermal conductivities. So, they are widely used in functional coatings, corrosion protection, high-temperature structural materials, microelectronics, and catalysts[1].
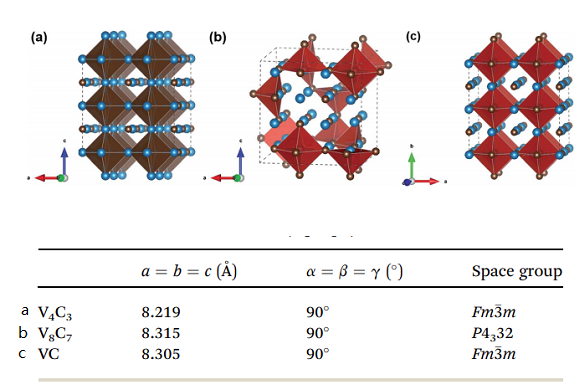
Vanadium forms the stoichiometric carbides such as VC and the non-stoichiometric cubic monocarbide VCy, which all have a B1 (NaCl) structure. A feature peculiar to the structure of non-stoichiometric VCy is the intrusion of carbon atoms in the octahedral interstices of the metallic sites. The carbon atoms may occupy only a fraction of the interstitial sites, and the rest are filled with structural vacancies. The carbon-atom vacancies arranged randomly on the fcc carbon sublattice are called disordered phases, while the ordered carbon vacancies take on a periodic arrangement. The disordered state of non-stoichiometric carbides is in thermodynamic equilibrium only at a high temperature, whereas the ordered state is in thermodynamic equilibrium at a temperature below 1300K. The disorder–order phase transition in the vanadium carbides and a cubic-ordered V8C7 phase can arise in the VCy carbide over the range VC0.86–VC0.88. The low-temperature phase V8C7 does not have an fcc structure; it crystallizes in the space group P4332, with the vanadium atoms occupying positions slightly off the ideal fcc positions and the carbon atoms having an ordered arrangement on the interstitial sites.
The crystal structure of VC belongs to the cubic rock salt family (NaCl type, Fm3m), which contains 8 atoms (4 V atoms, 4 C atoms) per unit cell. The emergence of natural carbon vacancies in V8C7 alters the space group to P4332, and the number of atoms counts up to 60 (32 V atoms, 28 C atoms) in one unit cell. But the structure remains cubic in general. To compare these compounds effectively, 2×2×2 supercell for V4C3 (32 V atoms, 24 C atoms) and VC (32 V atoms, 32 C atoms), one unit cell for V8C7 (32 V atoms, 28 C atoms) are used in the calculations such that each block contains the same amount of V atoms for all these three materials, as shown in Fig. 1. One can see that V and C atoms form VC6 octahedral structure. V4C3 and V8C7 have 8 C atoms and 4 C atoms vacancies per block, respectively. Tables 1 and S1† illustrate vanadium carbides' lattices and site information [2]. The lattice parameters of V4C3, V8C7, and VC are 8.219 Å, 8.315 Å, and 8.305 Å, respectively, which are in good agreement with theoretical and experimental works. Due to the appearance of artificial carbon vacancies, the lattice constants of V4C3 are smaller than VC. On the other hand, the formation of natural carbon vacancies changes the space group in the actual material V8C7, which produces a slightly larger lattice constant.
References
[1] X. Chong. “The effects of ordered carbon vacancies on stability and thermo-mechanical properties of V8C7 compared with VC.” Scientific Reports 6 1 (2016).
[1] Wan, Jing et al. “First-principles study of vanadium carbides as electrocatalysts for hydrogen and oxygen evolution reactions†.” RSC Advances 64 (2019): 37467–37473.
);