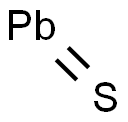The Structure and Uses of Lead(II) sulfide
Lead(II) sulfide (PbS), also known as galena, is a significant inorganic compound that serves as the principal source of lead and an important material in various technological applications.

Chemical Structure
The molecular formula for lead(II) sulfide is PbS. It consists of lead cations (Pb^2+) and sulfide anions (S^2-) in a stoichiometric ratio of 1:1. The crystal structure of galena is cubic, specifically belonging to the space group Fm-3m with a lattice parameter that typically ranges around 5.93 Å. The bonding between lead and sulfur atoms is predominantly ionic with some covalent character due to the polarizability of both ions. Each lead ion is surrounded by six sulfur ions at the corners of an octahedron, while each sulfur ion is similarly coordinated by six lead ions.
The Lead(II) sulfide molecule contains one non-hydrogen bond which is also a double bond, reflecting its binary composition comprising one atom each of lead and sulfur.
Physical Properties
Lead(II) sulfide has several notable physical properties:
Color: Pure galena exhibits a shiny metallic luster with a lead-gray color.
Specific Gravity: It has a high specific gravity between 7.2 and 7.6 g/cm³.
Hardness: On Mohs scale, its hardness varies from 2.5 to 2.75.
Semiconducting Material: It exhibits intrinsic semiconductor properties with a narrow band gap.
Uses
Ore Extraction
Galena's most significant role historically has been as the principal ore for extracting metallic lead through processes like smelting.
Semiconductor Applications
Due to its semiconducting nature, PbS has found use in electronic devices such as photodetectors and infrared detectors since it can respond to infrared light wavelengths ranging from about 0.7 µm up to about 3 µm.
Infrared Detectors
Lead(II) sulfide’s sensitivity to infrared light makes it useful in military applications for night vision equipment as well as in astronomy for detecting celestial bodies emitting infrared radiation.
Solar Cells
Advancements have shown PbS can be used in quantum dot solar cells due to its ability to absorb sunlight efficiently.
Optoelectronic Devices
Its electrical properties make it suitable for use in optoelectronic devices which require materials capable of converting light into electricity or vice versa.
Environmental Considerations
While PbS has many practical uses, it must be handled with care due to the toxicity associated with lead compounds which can pose environmental health risks if not managed properly during mining, processing, and waste disposal operations.
In summary, Lead(II) sulfide stands out not only for being the primary source of metallic lead but also for its specialized roles in modern technology due to its unique semiconductive and optoelectronic characteristics.
);You may like
Related articles And Qustion
See also
Lastest Price from Lead(II) sulfide manufacturers
US $0.00-0.00/KG2024-09-04
- CAS:
- 1314-87-0
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 10000KG
US $0.00-0.00/KG2022-10-11
- CAS:
- 1314-87-0
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton