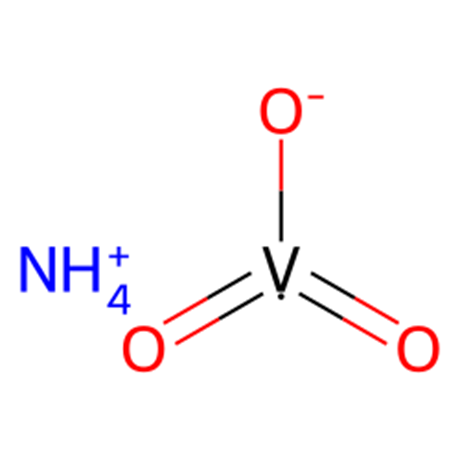
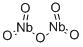The niobium oxide nanomaterials
Niobium pentoxides show high potential as acid catalysts for the sustainable production of fuels and chemicals from inedible biomass sources, but their performance needs to be improved to compete with catalysts currently used in industry. Niobium oxides' physical and chemical properties, including the acid properties, depend on their structure. Nb2O5 can be amorphous or crystalline, with many crystalline structures reported in the literature in which the main basic structural units are generally NbO6 octahedra, linked through corner- or edge-sharing. The structural diversity is attributed to the many different arrangements of these building blocks that can lead to the Nb2O5 stoichiometry. Additionally, phase mixtures and defects (e.g. atom vacancies or incorporation of additional atoms) frequently occur in niobium oxides, further contributing to the structural variety.
The solvothermal reaction of NbCl5 in acetophenone was used for synthesizing three niobium oxide nanomaterials: niobium oxide nanoparticles (Nb2O5-NP), spheres (Nb2O5-NS), and nanoparticles supported on holey graphene oxide (Nb2O5-hGO).
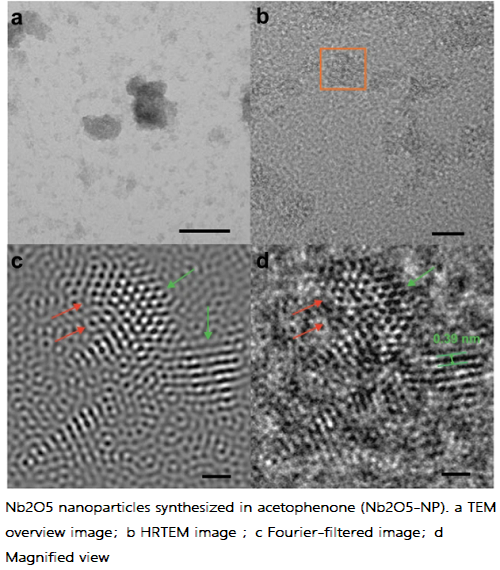
The reaction of NbCl5 with acetophenone produces platelet-like nanoparticles with ultrasmall dimensions within the range of 1.5–4 nm, which tend to form small agglomerates due to the absence of surfactant molecules in the synthesis. High-resolution transmission electron microscopy (HRTEM) images reveal the presence of lattice fringes, indicative of crystallinity, and defects in the particles, such as irregular surface boundaries and structural distortions.
Niobium oxide spheres (Nb2O5-NS) were produced by the reaction of NbCl5 with acetophenone in the presence of Co2+ ions, which were found previously to lead to the assembly of Co-doped ZnO nanocrystals synthesized in benzyl alcohol. Accordingly, the Co2+ ions were used to promote the assembly of the small niobium oxide nanoparticles and potentially increase their surface area, which is beneficial for catalysis. Cobalt was not detected in the final product, indicating that it did not react to form an oxide.

Niobium oxide nanoparticles were also synthesized at the surface of holey graphene oxide (Nb2O5-hGO). The figure shows TEM and HRTEM images of the composite, consisting of small oxide particles with sizes between ca. 2 and 4 nm well dispersed on the surface of the hGO support. Some lattice fringes are seen in the HRTEM image, reflecting crystallization.
References
[1] Kai Skrodczky. “Niobium pentoxide nanomaterials with distorted structures as efficient acid catalysts.” Communications Chemistry (2019): 1–11.
);You may like
See also
Lastest Price from Niobium oxide manufacturers

US $5.00-2.00/KG2024-09-19
- CAS:
- 1313-96-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000kg

US $0.00-0.00/KG2024-08-27
- CAS:
- 1313-96-8
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 10000KG