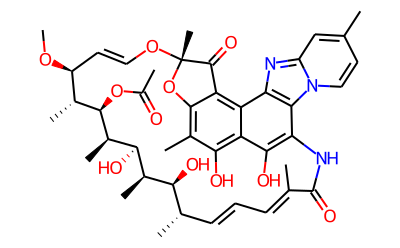
The Biological activity of Rifaximin
Introduction
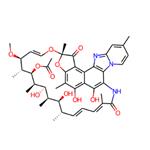
Rifaximin is a broad-spectrum antibiotic from the rifamycin family that hinders bacterial gene expression in both gram-positive and gram-negative organisms. It has proven to be remarkably effective in treating bacterial infections of the intestines with minimal side effects. Due to its low systemic absorption, the risk of adverse events associated with its administration is insignificant, indicating a high therapeutic index. Furthermore, its near non-absorbability results in the high bioavailability of this drug within the gastrointestinal tract. It also shows promising minimal inhibitory concentration values for many pathogenic microbes. It has been approved by the United States Food and Drug Administration (FDA) for managing traveler's diarrhea.
Uses
Rifaximin was first approved in Italy in 1987 and afterwards in several countries for the treatment of various gastrointestinal diseases and, in May 2004, approved by the US Food and Drug Administration for treating traveler's diarrhea caused by noninvasive strains of Escherichia coli. Rifaximin obtained from the Food and Drug Administration and most of the major European countries in 2010 and 2012, respectively, a new labelling for reduction of the risk of recurrence of overt hepatic encephalopathy in patients with advanced liver disease aged 18 years or older.
Antimicrobial effects
Rifaximin is a powerful antimicrobial in vitro, with a broad spectrum of antibacterial activity against Gram-positive and -negative, aerobic and anaerobic bacteria, including ammonia-producing species. Traditional MIC breakpoints for rifampin, set by the Clinical and Laboratory Standards Institute, based on drug plasma levels, have no relevance to rifaximin in treating enteric infection, being a minimally absorbed antibiotic. Based on clinical experience, an arbitrary breakpoint was set at 32 μg ml−1.
MIC50 and MIC90 values of rifaximin, determined against many different enteropathogens isolated in different countries, were mainly between 8–64 and 16–128 μg ml−1, respectively. Therefore, rifaximin showed intermediate in vitro activity compared with other antimicrobial agents such as nalidixic acid and ciprofloxacin. Moreover, rifaximin showed good in vitro activity against several enteropathogens, such as E. coli, Shigella spp and Salmonella spp. This is in accordance with the clinical studies that clearly show the effectiveness of the compound in the treatment of traveler's diarrhea. Rifaximin is also effective against Enterohemorrhagic E. coli causing diarrhea, bloody diarrhea and hemolytic-uremic syndrome by inhibition of the production and release of Shiga toxin, the major virulence factor of Enterohemorrhagic E. coli involved in the pathogenesis of hemolytic-uremic syndrome.
Prevention of bacterial adherence to gut mucosa
Rifaximin affects epithelial cell physiology, resulting in altered infectivity by enteric pathogens and baseline inflammation, suggesting that rifaximin conferred cytoprotection against bacterial colonization and infection. In fact, rifaximin pretreatment of Enteroaggregative E. coli decreased bacterial adherence to epithelial cells (HEp-2 (laryngeal)) without affecting their viability. The mechanism likely involved direct rifaximin-mediated alterations of the HEp-2 cells, which, in turn, affected bacterial adherence (directly, indirectly or both). Furthermore, the observation that the effects were time- and concentration-dependent suggested that a rifaximin-mediated process altered cellular parameters important to Enteroaggregative E. coli adherence. Reduced mucosal attachment may, at least, partially explain the beneficial effects of rifaximin in gastrointestinal diseases.
Anti-inflammatory effect
Rifaximin is an intestine-specific human Pregnane X Receptor (PXR) agonist. Recent studies in mice have provided insight into a novel function for PXR in IBD. The mechanism of the protective effect of PXR activation on IBD is not fully elucidated but is due in part to the attenuation of nuclear factor (NF)-κB signalling that results in lower expression of proinflammatory cytokines as interleukin (IL)-10, IL-1β, tumour necrosis factor-α. Thus, PXR may be a novel target for IBD therapy, and rifaximin's potential therapeutic value in IBD may be partly due to its PXR activation properties.
Rifaximin efficacy in reducing NF-κB signaling in a PXR-dependent manner was demonstrated in primary human colon epithelial cells and human colon biopsies. The preventive and therapeutic role of rifaximin in experimental models of IBD (dextran sodium sulfate and 2,4,6-trinitrobenzene sulfonic acid mouse models) were also demonstrated in PXR-humanized mice, where rifaximin not only prevented IBD before an inflammatory insult but also decreased symptoms after the onset of colitis.
References
[1] Zarghuna Khan. “Effectiveness of Rifaximin on the Outcomes of Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.” Cureus 15 9 (2023): e44807.
[2] Fiorella Calanni. “Rifaximin: beyond the traditional antibiotic activity.” Journal of Antibiotics 67 9 (2014): 667–670.
);You may like
Related articles And Qustion
See also
Lastest Price from Rifaximin manufacturers

US $0.00-0.00/kg2024-09-18
- CAS:
- 80621-81-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 500 KGS
US $0.00-0.00/kg2024-09-06
- CAS:
- 80621-81-4
- Min. Order:
- 1kg
- Purity:
- 99.0%
- Supply Ability:
- 10tons