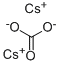
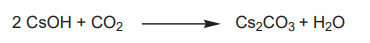
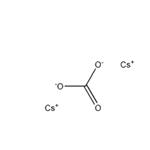
Synthesis of Cesium carbonate
Cesium carbonate is an inorganic compound. It is a white solid under normal temperature and pressure. It is easily soluble in water and quickly absorbs moisture when placed in the air. Cesium carbonate aqueous solution is strongly alkaline and can react with acid to produce corresponding cesium salt and water, and release carbon dioxide. Cesium carbonate is easy to transform, can be used as a precursor of other cesium salts, and is a widely used cesium salt.
Cesium carbonate can be used as a modified layer between the active layer of the solar cell and the Al electrode to improve the conversion efficiency of the device, generate Al-O-Cs compounds, reduce the series resistance of the battery, and improve the short-circuit current and photoelectric conversion efficiency of the solar cell, and Due to the compactness of the cesium carbonate layer itself, the stability of the device has also been significantly improved.
Preparation
Extracting high-purity cesium carbonate from pollucite:
(1) Grinding the ore: Use a ball mill to grind the ore to 120 mesh.
(2) Acidification reaction: Mix the ground pollucite ore powder in step (1) with 10 equivalents of concentrated hydrochloric acid (concentration of 32%), and heat to 100°C in a closed enamel reactor for reaction 2 Hour.
(3) Add water to the slurry after the reaction in step (2), mix with a liquid-to-solid ratio of 4:1, and heat to 80-100°C for leaching reaction for 1 hour.
(4) Add sodium hydroxide to the material after the reaction in step (3) to adjust the pH to 10 to precipitate impurities such as aluminum, silicon, calcium, and magnesium.
(5) Filter the material after step (4) with a filter press, and then wash the residue with water, and the ratio of liquid to solid of the washing residue is 1:1.
(6) After adjusting the components of the leachate in step (5), enter the continuous mixing and clarification extraction tank to extract with the special extractant "Second BAMBP", because this extractant has excellent selectivity for cesium and rubidium, and the separation coefficient is very large. The cesium is obtained by preferential extraction, and the cesium carbonate stripping liquid is obtained after stripping with carbon dioxide.
(7) The cesium carbonate stripping solution produced in step (6) is concentrated, and carbon dioxide is introduced for deep carbonization, the carbonization pressure is 0.2Mpa, and the carbonization time is 1 hour. The crystals are further concentrated and precipitated, and the crystals and the mother liquor are centrifuged.
(8) The crystalline product produced in step (7) is dissolved in pure water with a liquid-solid ratio of 2:1, filtered, and then concentrated and crystallized again, centrifuged to obtain secondary crystals.
(9) Put the secondary crystal obtained in step (8) into an oven for drying and crushing to obtain a cesium carbonate product with a purity of 99.99%. The total yield of cesium is 94.59%.
);You may like
Related articles And Qustion
Lastest Price from Cesium carbonate manufacturers

US $990.00-800.00/kg2024-09-19
- CAS:
- 534-17-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000
US $25.00/KG2024-09-19
- CAS:
- 534-17-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 1000kg