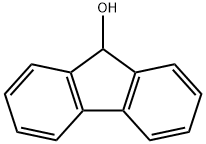
Synthesis and Thermodynamic property of 9-Fluorenol
General description
9-Fluorenol is crystalline powder, slightly soluble in chloroform and methanol. It is a wake stimulant and is considered the next generation of anti-lethargy drugs. It is also a potential environmental carcinogen.

Fig. 1 The structure of 9-Fluorenol.
Synthetic routes
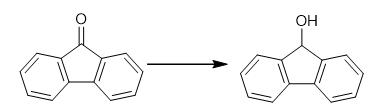
Fig. 2 The synthetic method 1 of 9-Fluorenol.
Add 9-fluorenone (0.8 mmol), a 0.2 M THF solution of phosphazene (80 μL, 0.016 mmol) and a 0.0762 M THF solution of boron catalyst (420 μL, 0.032 mmol) to a scintillation vial with magnetic stir bar in a glove box. Place the scintillation vial in a Parr reactor. Seal the reactor. Pressurize the reactor with hydrogen gas. Heat reaction mixture at 75°C (inside the reactor). Stir the reaction mixture (1000 rpm) for 20 hours at 75°C. Cool the mixture to room temperature and vent the Parr reactor to obtain 9-hydroxyfluoren. Analyze the reaction mixture by 1H NMR spectroscopy using CDCl3 as solvent [1].
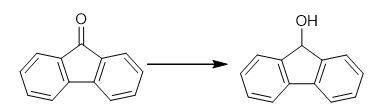
Fig. 3 The synthetic method 2 of 9-Fluorenol.
Dissolve catalyst (0.54 mg, 1.0 μmol) in Et2O (1.0 mL) in a 3.8 mL glass vial equipped with a stir bar in a glove box under N2 atmosphere. Add 9-Fluorenone (180.2 mg, 1.0 mmol) and pinacolborane (140.8 mg, 1.1 mmol). Allow the reaction mixture to stir at room temperature for 2 h. Expose the reaction to the air and evaporate the solvent. Detect the hydroborated product by GC-MS. Purify the crude reaction mixture through flash column chromatography with SiO2 using ethyl acetate/hexane (1:10, v/v) as eluent. 1H NMR (600 MHz, CDCl3) δ 7.64 (dd, J = 15.8, 7.7 Hz, 4H), 7.41 (t, J = 7.6 Hz, 2H), 7.34 (t, J = 7.6 Hz, 2H), 5.54 (s, 1H), 2.08 (s, 1H) ppm; 13C NMR (151 MHz, CDCl3) δ 145.7, 140.0, 129.1, 127.8, 125.2, 120.0, 75.2 ppm [2].
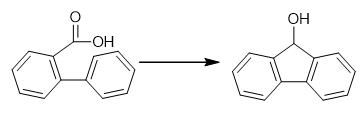
Fig. 4 The synthetic method 3 of 9-Fluorenol.
Dissolve acid derivative (2.02 mmol) in thionyl chloride (8 ml). Stir the resulting mixture for 4 hours. Remove excess thionyl chloride via trap to trap distillation. Add dichloromethane (20 ml) to the remaining brown solid. Add the resultant to the stirred solution aluminium trichloride (0.490 g, 6.06 mmol), observe a deep red colouration. Stir the reaction mixture for a further 2 hours. Add water (5 ml) to the mixture. Filter the mixture. Extract the resultant with a further portion of dichloromethane (3 x 10 ml). Combine the organic extracts. Dry the resultant over magnesium sulfate. Remove excess solvent under reduced pressure. Add lithium aluminium hydride (158 mg, 4.16 mmol) in one portion to an resulting ice-cooled solution (2.77 mmol) in anhydrous tetrahydrofuran (25 mL) under dynamic nitrogen at 0 °C. Stir the reaction mixture at 0 °C for one hour. Quench the resultant with water until observation of no further bubbling (ca. 3 mL). Filter the resulting white suspension. Wash the residue with ether (30 mL). Dry the filtrate over magnesium sulfate. Filter the mixture. Concentrate the resultant under reduced pressure to obtain fluorenol [3].
Thermodynamic Property
This report presents a study of the thermodynamic properties of 9-fluorenone and 9-fluorenol. The standard enthalpies of formation of 9-fluorenone and 9-fluorenol in the crystalline phase at T = 298.15 K were determined from the energies of combustion in oxygen as -(11.4 +/- 3.8) kJ.mol(-1) and -(66.3 +/- 2.9) kJ.mol(-1), respectively. Vapor pressures of the two compounds were measured at several temperatures using two different experimental methods (Knudsen effusion for crystalline-phase vapor pressures and a static method for crystalline- and liquid-phase vapor pressures), which yielded reliable values of the enthalpies of sublimation, vaporization, and fusion. The sublimation and fusion enthalpies were also determined using calorimetric methods. The enthalpies of sublimation at T = 298.15 K were derived from the static vapor pressure measurements as (95.1 +/- 0.5) kJ.mol(-1) for 9-fluorenone and (108.3 +/- 0.5) kJ.mol(-1) for 9-fluorenol. The results enabled the determination of the standard Gibbs energy of the aerobic reactions of oxidation of fluorene to 9-fluorenol and 9-fluorenone and of the oxidation of 9-fluorenol to 9-fluorenone. Values of the standard enthalpies of formation in the gas phase were also calculated [4].
References
[1] Mummadi S, Brar A, Wang G, et al. “Inverse” Frustrated Lewis Pairs: An Inverse FLP Approach to the Catalytic Metal Free Hydrogenation of Ketones[J]. Chemistry–A European Journal, 2018, 24(62): 16526-16531.
[2] Zhang G, Wu J, Zheng S, et al. Redox-noninnocent ligand-supported vanadium catalysts for the chemoselective reduction of C═ X (X= O, N) functionalities[J]. Journal of the American Chemical Society, 2019, 141(38): 15230-15239.
[3] George S R D, Elton T E, Harper J B. Electronic effects on the substitution reactions of benzhydrols and fluorenyl alcohols. Determination of mechanism and effects of antiaromaticity[J]. Organic & Biomolecular Chemistry, 2015, 13(43): 10745-10750.
[4] Monte M J S, Notario R, Calvinho M M G, et al. Experimental and computational study of the thermodynamic properties of 9-fluorenone and 9-fluorenol[J]. Journal of Chemical & Engineering Data, 2012, 57(9): 2486-2496.
);See also
Lastest Price from 9-Fluorenol manufacturers

US $50.00/kg2024-08-15
- CAS:
- 1689-64-1
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000

US $0.00/kg2024-08-14
- CAS:
- 1689-64-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 50000KG/month