Ramelteon: Pharmacodynamics and Pharmacokinetics
General Description
Ramelteon is a melatonin receptor agonist with high affinity for MT1 and MT2 receptors, displaying minimal affinity for MT3 receptors. It effectively modulates sleep patterns without significant impact on other physiological functions. Clinical studies demonstrate its efficacy in reducing sleep latency and improving sleep maintenance in patients with insomnia. In animal models, Ramelteon promotes sleep onset and duration without sedative or cognitive effects. Pharmacokinetically, it shows dose-proportional kinetics, primarily metabolized by CYP1A2. Potential drug interactions with enzyme inhibitors or inducers necessitate cautious coadministration. Overall, Ramelteon's selective binding properties and favorable pharmacodynamic profile make it a promising option for managing insomnia.

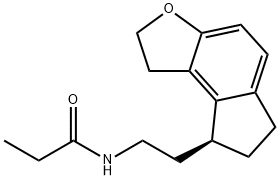
Figure 1. Ramelteon
Pharmacodynamics
Receptor Binding Properties
Ramelteon exhibits distinct receptor binding properties that contribute to its pharmacological profile. In vitro studies have demonstrated that Ramelteon has a high affinity for human MT1 and MT2 receptors, with dissociation constants (Ki) of 14.0 and 112 pmol/L, respectively. This affinity is significantly greater compared to its binding affinity for MT3 receptors, where the Ki is 2650 nmol/L. In comparison, melatonin, a related compound, shows a Ki of 0.0807 and 0.383 nmol/L for MT1 and MT2 receptors, respectively, and 24.1 nmol/L for MT3 receptors. This selective binding profile of Ramelteon suggests a strong preference for MT1 and MT2 receptors over MT3, emphasizing its role in modulating circadian rhythms without affecting melatonin receptors associated with other physiological functions.
Human Studies and Sleep Modulation
Clinical studies in humans further elucidate Ramelteon's pharmacodynamics, particularly its efficacy in treating insomnia. In a randomized, double-blind study involving patients with chronic insomnia, Ramelteon significantly reduced sleep latency compared to placebo. Administered over two consecutive nights, Ramelteon at doses of 4, 8, 16, or 32 mg shortened sleep latency by 22.9 to 24.3 minutes (p < 0.001). Moreover, it increased total sleep time and sleep efficiency, indicating its ability to improve sleep architecture without impacting wakefulness or sleep quality metrics significantly. These findings underscore Ramelteon's efficacy in promoting sleep onset and enhancing sleep duration in clinical settings.
Animal Studies and Neuropharmacology
In animal models, Ramelteon demonstrates potent sleep-promoting effects without influencing other behavioral parameters. Orally administered Ramelteon reduced latency to sleep onset and increased total sleep duration in young adult female macaques. In contrast, melatonin and zolpidem showed differing effects on sleep metrics, highlighting Ramelteon's unique pharmacodynamic profile. Furthermore, Ramelteon did not induce sedation or affect cognitive functions such as memory recall or alertness levels, distinguishing it from other sleep aids like zolpidem which are associated with sedative effects. These animal studies reinforce Ramelteon's specificity in modulating sleep behaviors while preserving cognitive functions.
Ramelteon's pharmacodynamics are characterized by its selective binding to MT1 and MT2 receptors, leading to effective modulation of sleep patterns without significant effects on other neuroreceptor systems. Clinical and preclinical studies consistently demonstrate its efficacy in reducing sleep latency and improving sleep maintenance, making it a valuable option for managing insomnia without the risks associated with traditional sedative-hypnotic agents. Future research may further elucidate its precise mechanisms of action and potential applications in treating sleep disorders beyond insomnia. 1
Pharmacokinetics
Absorption and Distribution
Ramelteon, when administered orally, is quickly and extensively absorbed in the body. However, due to significant first-pass metabolism, the absolute bioavailability of a single oral 16mg dose is less than 2%. In a dose-escalation study involving 60 volunteers, the area under the plasma concentration-time curve (AUC) and maximum plasma concentration (Cmax) values of ramelteon were found to be dose proportional. The time to reach peak concentration (Cmax) remained relatively constant across different doses, indicating predictable pharmacokinetics at various dose levels. 2
Metabolism and Excretion
Upon oral administration, ramelteon undergoes extensive hepatic metabolism primarily involving cytochrome P450 (CYP) enzymes, particularly CYP1A2, with minor contributions from CYP2C and CYP3A4 subfamilies. The major active metabolite, M-II, exhibits significantly higher systemic exposure compared to ramelteon itself, with a longer half-life of 1.2 hours. The elimination process involves the formation of four main metabolites, with oxidative pathways leading to metabolites with varying pharmacological activities. The majority of the administered dose is excreted in the urine, with minimal urinary excretion of unchanged ramelteon. 2
Potential Drug Interactions
Given that ramelteon is primarily metabolized by CYP1A2, potential drug interactions can occur with inhibitors or inducers of this enzyme system. For example, coadministration of ramelteon with fluvoxamine, a potent CYP1A2 inhibitor, significantly increases the AUC of ramelteon. Conversely, rifampicin, a CYP inducer, reduces the systemic exposure of ramelteon. Other medications such as ketoconazole and fluconazole affect ramelteon's exposure to varying degrees. Understanding these interactions is crucial in clinical practice to optimize the efficacy and safety of ramelteon therapy. 2
Reference
1. McGechan A, Wellington K. Ramelteon. CNS Drugs. 2005; 19(12): 1057-1067.
2. Takeda Pharmaceuticals America Inc.RozeremTM (ramelteon) tablets:prescribing information. Available from URL: http://www.rozerem.com/pi.pdf
);Lastest Price from Ramelteon manufacturers

US $100.00/kg2024-09-19
- CAS:
- 196597-26-9
- Min. Order:
- 1kg
- Purity:
- 99.9%
- Supply Ability:
- 20tons

US $3.00-1.00/kg2024-04-16
- CAS:
- 196597-26-9
- Min. Order:
- 1kg
- Purity:
- 99.9%
- Supply Ability:
- 10 tons