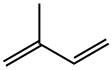
How to synthesize Isoprene?
Isoprene is a colorless, volatile, and irritating oily liquid at room temperature. It is almost insoluble in water but soluble in most organic solvents such as hydrocarbons, alcohols, and ethers. Isoprene can form binary and ternary azeotropes with various organic substances. Isoprene is chemically active and easy to polymerize.
synthesis method
The commercial processes for isoprene production include the extraction method (recovery from C5 fraction), dehydrogenation of isopentane or isopentene, the reaction of isobutylene with formaldehyde, and the acetylene–acetone route. Here, only the acetylene–acetone route for isoprene production is described. Acetone underwent an ethynylation reaction with acetylene to yield methylbutynol, followed by partial hydrogenation to 2-methyl-3-buten-2-ol and, finally, Dehydration to the desired product. The reaction equations are as follows:

Ethynylation
Three parts of the strong basic anion exchange resin Amberlite IRA400 were suspended in 30 parts of 7% sodium hydroxide solution to change to OH type. After washing to neutral with water, the resin was suspended in methanol, and after 15–60 min, methanol was discharged. The treatment with methanol was repeated as described above until the water content of the effluent methanol was less than 0.1% and the water content of the ion exchange resin was less than 0.2%. The tubular reactor was filled with the ion exchange resin, and then acetone was saturated with acetylene, and liquid ammonia was introduced into the reactor at 2.5 MPa and 40 °C. Excess acetylene in reactants was used to reduce the side reaction of acetone. After ethynylation, the reaction solution was subjected to a flash evaporation at 50 °C. Ammonia and the unreacted acetylene were recovered and recycled. The unreacted acetone was recovered by distillation and recycled. The remaining solution was distilled to obtain methylbutynol. The conversion of acetone was 90%, and the selectivity to methylbutynol was 95%. After continuous operation for 25 days, the ion exchange resin must be regenerated.
Selective hydrogenation
The methylbutynol and hydrogen were introduced into the hydrogenation reactor filled with a Pd/CaCO3 catalyst. The hydrogenation was carried out at 50–80 °C and under 0.5 MPa. The main by-product is methylbutanol. In order to suppress the formation of by-products, a small amount of zinc salt is added as an inhibitor. The conversion of the reaction was 100%, and the selectivity to methyl butanol was between 98% and 99%. The reaction mixture was separated in a gas–liquid separator. The unreacted hydrogen was recycled. The liquid was evaporated and finally sent to a dehydration reactor.
Dehydration
The azeotrope of methyl butanol and water was pumped to a fixed-bed dehydration reactor filled with an alumina catalyst. The Dehydration of methyl butanol was carried out at 260–300 °C and under atmospheric pressure. Methyl butanol was converted from 88% to 98%, and the selectivity to isoprene was more than 98%. The dehydrated product was washed with water in a washing column to remove a small number of water-soluble substances and then sent to two distillation towers in series to remove the light and heavy fractions, respectively. Isoprene of polymerization grade was obtained at the top of the heavy fraction removing tower.
);You may like
See also
Lastest Price from Isoprene manufacturers

US $1350.00/T2024-08-06
- CAS:
- 78-79-5
- Min. Order:
- 1T
- Purity:
- 99%
- Supply Ability:
- 600T
US $100.00-1.00/KG2024-03-25
- CAS:
- 78-79-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available