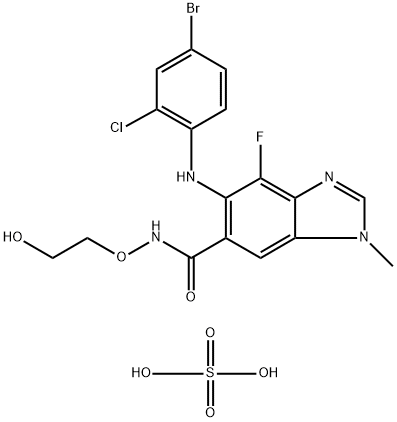
How is Selumetinib Sulfate Synthesised?
Synthesis of Selumetinib Sulfate
Selumetinib Sulfate was prepared by a two-step chemical reaction by first synthesising Selumitinib Benzimidazole intermediates using 2,3,4-trifluorobenzoic acid as starting material. The specific synthesis steps are as follows:
Step 1: Preparation of Selumitinib Benzimidazole
A synthetic sequence described jointly by Array BioPharma and AstraZeneca has been disclosed, and this route started from 2,3,4-trifluorobenzoic acid 345. Nitration of 345, SNAr amination of 346, esterification of the carboxylic acid 347, and a final SNAr amination of 348 provided the requisite core functionality to rapidly complete the synthesis. Reduction of the nitro group of 349 with Pd(OH)2 and in situ condensation with formic acid smoothly provided 1H-benzoimidazole 350 in good yield. Sequential halogenation with NBS and NCS gave the 4-bromo-2-chloroanilino moiety 352. Methylation of the 1H-benzoimidazole provided 353 in modest yield, likely due to incomplete regioselectivity.
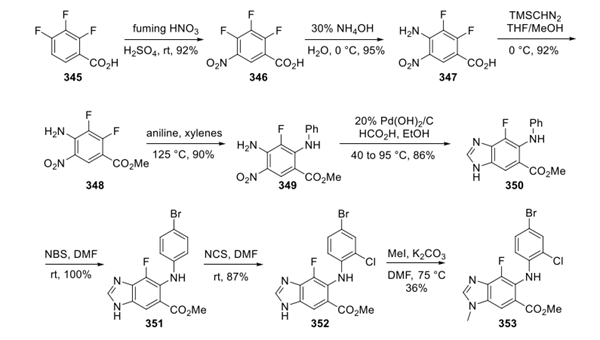
Step 2: Preparation of Selumetinib Sulfate
Saponification of the ester within 353, followed by EDCI/HOBt-mediated amidation with hydroxylamine 355, gave acetamide 356 in good yield. Acid hydrolysis of the vinyl ether quantitively afforded alcohol 357. Finally, salt formation with H2SO4 in 2- butanone gave selumetinib as the sulfate salt.