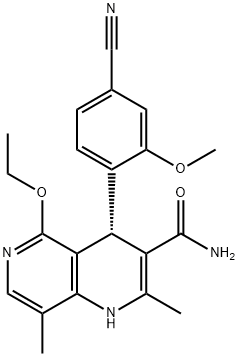
Finerenone: Synthesis and Introduction
Synthesis of Finerenone
Finerenone is prepared by a series of reactions with benzaldehyde and pyridyl chloride as starting materials. The specific synthesis steps are as follows:

Benzaldehyde 20.1 was condensed with acetoacetamide under Knoevenagel conditions to furnish an undetermined mixture of enones 20.2 in excellent yield. Concurrently, pyridyl chloride 20.3 wassubjected to strongly basic conditions at a high temperature, followed by a chilled strong acid, which furnished pyridone 20.4 as a filterable solid. Addition of 20.2 to 20.4 in heated isobutanol resulted in a condensation−cyclization reaction to afford azacycle 20.5 in a good yield. Conversion of the pyridone to the corresponding ethyl ether was accomplished upon exposure to 1,1,1-triethoxyethane under acidic conditions. While some disclosed preparations of finerenone separate the racemic 20.6 by chromatography, a more recent patent reported a classical resolution using various tartaric acid-derived salts. In one example, treatment of 20.6 with di-p-toluoyl-D-tartaric acid in ethanol and water provided the desired enantiomer in 78% ee, which could be upgraded by reslurry in ethanol and water to 99% ee to provide 20.7 in~41% yield. Adjustment of pH using sodium carbonate in water and ethanol gave the free base of finerenone in 97% yield.
Introduction of Finerenone
Finerenone, or BAY94- 8862, is a non-steroidal mineralocorticoid receptor (MR) antagonist approved by the USFDA in July 2021 and the EMA and the Japanese Ministry of Health, Labour, and Welfare in 2022. The drug's indication is to reduce the risk of sustained estimated glomerular filtration rate decline, end-stage renal disease, cardiovascular death, nonfatal myocardial infarction, and hospitalization for heart failure in adult patients with CKD associated with type 2 diabetes (T2D).
Finerenone, which was developed by Bayer, exhibits potency and selectivity for MR, where it blocks sodium resorption and overactivation, while having minimal effects on serum potassium and kidney function. Unlike the related drugs spironolactone and eplerenone, finerenone has demonstrated a favorable benefit−risk profile, offering an effective new treatment for patients with CKD associated with T2D. Increases in serum potassium are reportedly predictable and are expected to be manageable in the context of clinical practice.
);You may like
Related articles And Qustion
See also

US $0.00-0.00/kg2024-09-14
- CAS:
- 1050477-31-0
- Min. Order:
- 1kg
- Purity:
- 99%,single impurity<0.1
- Supply Ability:
- 1 ton

US $0.00/kg2024-09-09
- CAS:
- 1050477-31-0
- Min. Order:
- 1kg
- Purity:
- 98% HPLC
- Supply Ability:
- 100 kgs