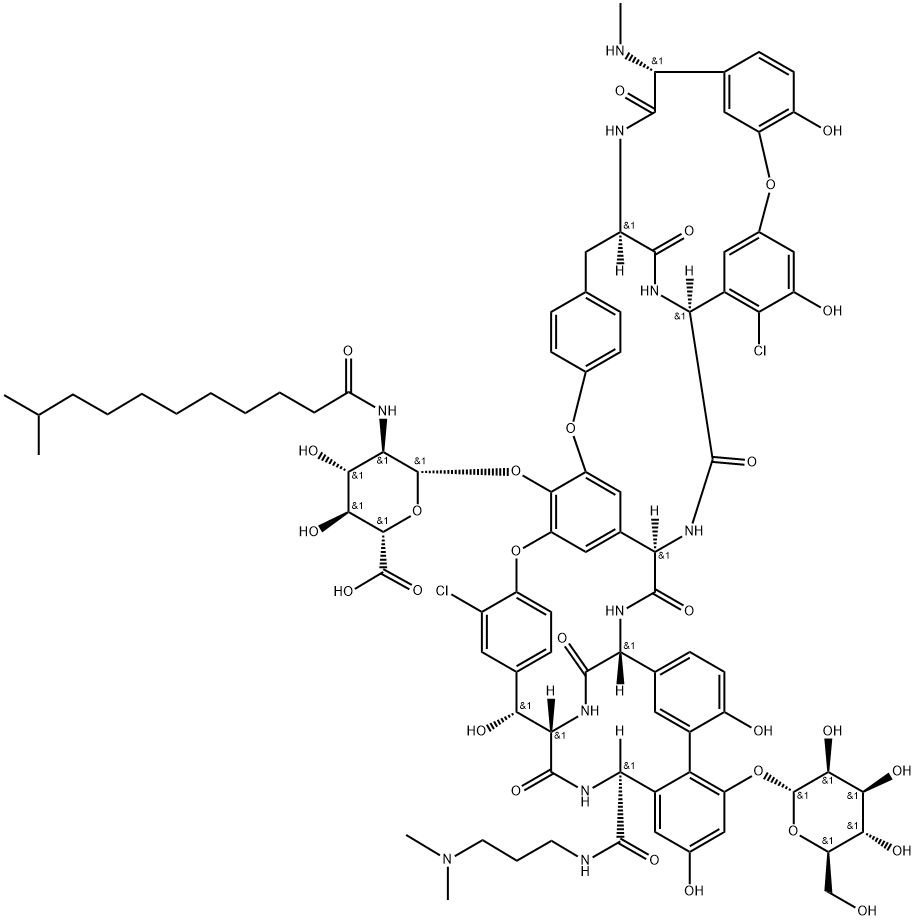
Dalbavancin---semisynthetic lipoglycopeptide
DESCRIPTION
Dalbavancin (Zeven, Pfizer Inc.) is a semisynthetic lipoglycopeptide with an extended half-life that enables once-weekly dosing. It has potent in vitro activity against most Gram-positive organisms with lower minimum inhibitory concentration (MIC) values than vancomycin and other investigational lipoglycopeptides (i.e., oritavancin, telavancin). It is also active against drug-resistant pathogens of concern such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE), except for strains exhibiting vanA resistance.
Derived from the naturally occurring A-40926 (a family member of teicoplanin), dalbavancin maintains the peptide backbone that is characteristic of glycopeptides. It has several structural changes, which include a 3,3-dimethylaminopropylamide in place of a peptide carboxy group, which increases antistaphylococcal potency, particularly against coagulase-negative staphylococci (CoNS). Its lipophilic side chain increases protein binding and thus provides the long half-life (t1/2) typical of teicoplanin-type derivatives. The side chain also provides additional mechanisms that enhance dalbavancin’s interaction to its target binding site.
MECHANISM OF ACTION
Like other glycopeptides, dalbavancin inhibits the final stages of peptidoglycan synthesis by forming a binding pocket with the D-alanyl-D-alanine terminus of peptidoglycan precursors. The complex created between the heptapeptide backbone and the D-alanyl-D-alanine dipeptide blocks access for transglycosylases and transpeptidases, enzymes that are necessary to pursue polymerization and cross-linking. As a result, the nascent peptidoglycan chain is halted from developing further, leaving cells vulnerable to rupture from changing internal osmotic pressure.
The addition of a lipophilic side chain seems to afford dalbavancin more ways to improve the interaction with D-alanyl-D-alanine peptides. It is hypothesized to allow for dimerization (like vancomycin) and membrane anchoring (like teicoplanin), which increases dalbavancin’s binding affinity to the target site. Homodimers formed between glycopeptide molecules lock the binding pocket into a prime position to facilitate cooperative binding. Membrane anchoring helps localize dalbavancin nearer to its target. These features are considered to contribute to dalbavancin’s antimicrobial activity. Although it was initially designed to be active against vanAresistant strains, dalbavancin fails to do so. Glycopeptides would require specific changes to its core backbone in order to permit binding to the D-alanyl-D-lactate carried by vanA enterococci. Telavancin is capable of overcoming vanA resistance due to a second mechanism of action, cellular membrane depolarization, and permeabilization, which dalbavancin does not display.
);Lastest Price from Dalbavancin manufacturers

US $0.00/g2024-09-11
- CAS:
- 171500-79-1
- Min. Order:
- 1g
- Purity:
- More Than 99%
- Supply Ability:
- 100kg/Month

US $0.00/kg2024-04-12
- CAS:
- 171500-79-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000ton