Classical Synthesis and Recent Synthetic Advances in Ibuprofen
Inflammation is a natural defense response of the immune system to non-self-recognition or unusual self-abnormality. Although this crucial process protects our body from unusual disorders, it sometimes causes unwanted physical symptoms such as pain and heat. Ibuprofen was developed in the 1960s and was valued at around 300 million US dollars in 2020. In addition, it was prescribed more than 2.4 million times in the USA in 2018 and was the most prescribed NSAID in that year.
Classical Synthesis
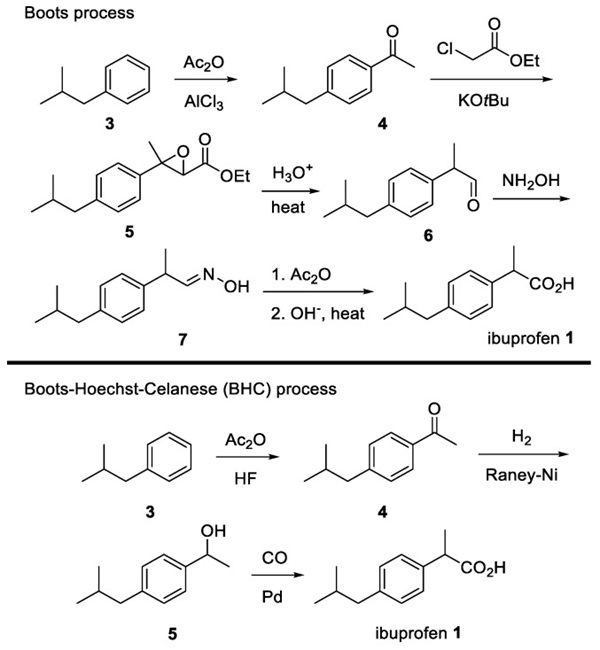
Scheme 1. Classical synthesis of Ibuprofen.
The classical synthesis of ibuprofen is shown in Scheme 1. When the Boots Pure Drug Company developed ibuprofen in 1961, it was prepared in six steps, including the use of toxic aluminum chloride in the early stage. However, in 1992, the Hoechst Company protocol improved this procedure by using recyclable hydrogen fluoride as an alternative to aluminum chloride. Moreover, the synthesis was accomplished using a simple carbon monoxide (CO) insertion method without additional hydrolysis or dehydration. With a three-step procedure, ibuprofen can be supplied worldwide. However, there is still an unmet need for a simpler, more efficient, and stereospecific route.
Recent Synthetic Advances
McQuade group used trifluorosulfonic acid as a reaction catalyst instead of the conventional reagent, aluminum chloride, to achieve continuous-flow synthesis. Aluminum byproducts are incompatible in further steps. The next step was the 1,2-aryl migration reaction. After the model study and careful survey of reaction conditions, substrate ketone 9 could be quantitatively converted into methyl ester 10. Finally, saponification of the methyl ester functionality afforded ibuprofen as a light orange solid. It is noteworthy that the reaction could be completed in 10 min using a flow reactor with a 68% overall yield (51% after recrystallization). Moreover, the simplicity and efficiency of this synthetic process makes it likely to satisfy the unmet need for improved ibuprofen synthesis.
Another approach employing continuous-flow synthesis was reported in 2019. This synthesis involves direct alkylation of the benzylic anion via 'superbase'-mediated deprotonation. Starting from p-xylene, in situ generated 'superbase' from KOtBu and tBuLi afforded the desired benzylic anion to meet with adequate electrophiles sequentially. After 10 min, the process, which employed the use of MeOTf, iPrI, and CO2, produced 2.3 g ibuprofen (50% in three steps). The readily available starting material and repeated usage of the mixed base increases the cost-effectiveness of this approach. However, the strong basicity of this procedure still requires improvement for industrial purposes.
Reference
[1] Recent Advances in the Synthesis of Ibuprofen and Naproxen. doi:10.3390/molecules26164792
);Related articles And Qustion
Lastest Price from Ibuprofen manufacturers

US $0.00-0.00/Kg/Bag2024-09-19
- CAS:
- 15687-27-1
- Min. Order:
- 1Kg/Bag
- Purity:
- 0.99
- Supply Ability:
- 20 tons

US $30.00-10.00/kg2024-09-19
- CAS:
- 15687-27-1
- Min. Order:
- 10kg
- Purity:
- 99%
- Supply Ability:
- 100000kg