Applications of 1,2,5-Oxadiazole
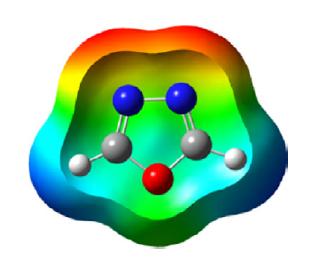
1,2,5-Oxadiazole is a five-membered, heteroaromatic, planar heterocycle comprised of one oxygen atom with two vicinal nitrogen atoms (N-O-N) and two carbon atoms at the 3- and 4-positions of the ring. It is a π-excessive heterocycle also referred to by its trivial name furazan and corresponding 2-N-oxide as furoxan. The Bird unified aromaticity index for 1,2,5-oxadiazole is 53 and is closer to isoxazole (52). The ionization energy of oxadiazole is 11.79 eV and the dipole moment is 3.38 D greater than isoxazole. The π electron density of the nitrogen atom is greater than the oxygen and carbon atoms as depicted in the following scheme.
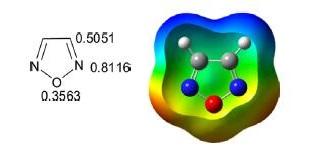
The 6π electrons are delocalized over the ring. The pKa (≈ −5.0) of 1,2,5-oxadiazole is less than isoxazole (pKa −2.97), which indicates that it is less basic than isoxazole.
Synthesis
There are three main approaches for the construction of 1,2,5-oxadiazoles: (1) dehydration of dioximes, (2) deoxygenation of 1,2.5-oxadiazol-2-oxide, and (3) through-ring transformation reactions.
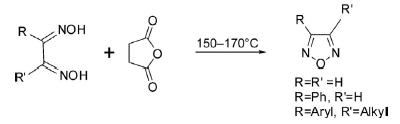
1. Dehydration of Dioximes
The parent 1,2,5-oxadiazole has been prepared by dehydration of glyoxaldioxime obtainable from the reaction of glyoxal with hydroxylamine by heating with succinic anhydride at 150–170°C or using SOCl2 as a dehydrating agent, and has been found highly suitable yield-wise.
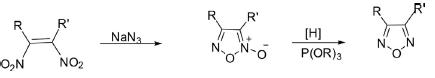
2. Deoxygenation of 1,2,5-Oxadiazol-5-Oxide
1,2,5-Oxadiazoles bearing different substituents, including alky, aryl, acyl, cyano, and amino groups, have been synthesized by reduction of 1,2,5-oxadiazole-2N-oxide with trialkylphosphite.
Physical Properties
The parent 1,2,5-oxadiazole is a colorless liquid with an mp of −28°C and a bp of 98°C, soluble in water and stable at room temperature. Its density is 1.168 g/cm.3
Applications of 1,2,5-Oxadiazoles
1,2,5-Oxadiazole derivatives are found to be potent inhibitors of indoleamine 2,3-dioxygenase and are useful for the treatment of cancer and other disorders. They are also useful as a new class of SENP2 inhibitors and can be used for the development of novel therapeutic agents for various diseases targeting SENPs. 1,2,5-Oxadiazole-2-oxides are used as a source of NO in biological studies. 4-Amino-1,2,5-oxadiazole-2-oxide-3-carboxylic acid and azo derivatives have been studied for their vasodilating properties.
The fluorogenic property associated with 1,2,5-oxadiazoles is well known and is used in light-emitting devices. These compounds emit the fluorescence of an orange to red color in solution and solid states. 1,2,5-Oxadiazole-2-oxide and benzo[c][1,2,5]oxadiazole-N-oxide derivatives are found to display herbicidal activity. 7-Amino-4,6-dinitrobenzofuroxan and 5,7-diamino-4,6-dinitrobenzofuroxan are high energetic material and perspective explosives.
);