Aluminum Ammonium Sulfate: A Versatile Compound in Chemistry
Introduction
Aluminum ammonium sulfate, or ammonium alum, is a widely used chemical compound in various industrial and laboratory applications. Its unique properties and versatility make it a subject of interest for chemists and professionals in related fields. This article aims to provide a comprehensive overview of aluminum ammonium sulfate, including its properties, main components, applications, and storage methods.

Figure 1 Characteristics of Aluminum ammonium sulfate
Properties
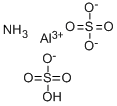
Aluminum ammonium sulfate is a double sulfate salt It appears as a white crystalline solid and is highly soluble in water. The compound crystallizes in the cubic crystal system and forms large, transparent crystals often used in laboratory demonstrations and experiments.
One of the notable properties of aluminum ammonium sulfate is its astringent taste and odorless nature. It has a melting point of approximately 93.5°C (200.3°F), and upon heating, it decomposes to form ammonia, sulfur dioxide, and aluminum oxide. The solubility of aluminum ammonium sulfate in water increases with temperature, making it easier to dissolve in hot water than in cold.
Main Components
The main components of aluminum ammonium sulfate are aluminum sulfate and water of crystallization. The presence of these components gives the compound its unique properties and versatility in various applications.
Aluminum Sulfate: This component provides the aluminum ions needed for various chemical reactions. Aluminum sulfate is known for its use in water purification, paper manufacturing, and as a mordant in dyeing processes.
Ammonium Sulfate: Ammonium ions from this component play a crucial role in fertilizer production and as a nitrogen source in agriculture. Ammonium sulfate is also used in protein purification by precipitation.
Water of Crystallization: The 12 molecules of water of crystallization in aluminum ammonium sulfate contribute to its crystalline structure and solubility. These water molecules are essential for maintaining the compound's stability and form.
Applications
Aluminum ammonium sulfate has a wide range of applications in various industries due to its chemical properties and effectiveness. Some of the key applications include:
Water Treatment: Aluminum ammonium sulfate is used as a coagulant in water treatment processes to remove impurities and suspended particles. It helps in the aggregation of fine particles, making them easier to filter out.
Dyeing and Printing: In the textile industry, aluminum ammonium sulfate serves as a mordant, helping to fix dyes onto fabrics. This enhances the colorfastness and vibrancy of the dyes used in textile printing and dyeing.
Food Additive: It is used as a food additive, particularly in baking powders and pickling processes. In baking, it acts as a leavening agent, while in pickling, it helps maintain the crispness of vegetables.
Laboratory Use: Aluminum ammonium sulfate is commonly used in laboratories for various experiments, including crystallization demonstrations and as a reagent in chemical reactions.
Cosmetics: In the cosmetic industry, it is used in products like deodorants and antiperspirants for its astringent and antibacterial properties. It helps reduce perspiration and control odor.
Pharmaceuticals: The compound is also used in the pharmaceutical industry for the preparation of certain medications and vaccines, taking advantage of its stabilizing properties.
Storage Methods
Proper storage of aluminum ammonium sulfate is essential to maintain its stability and effectiveness. The compound should be stored in a cool, dry place away from moisture and incompatible substances. Some key storage considerations include:
Temperature Control: Aluminum ammonium sulfate should be stored at room temperature, away from heat sources. High temperatures can cause the compound to decompose, releasing ammonia and other gases.
Humidity Control: Moisture can cause the compound to clump and lose its crystalline structure. It should be stored in airtight containers to prevent exposure to humidity.
Incompatibility: The compound should be kept away from strong acids and bases, as well as oxidizing agents. These substances can react with aluminum ammonium sulfate, leading to hazardous conditions.
Labeling and Safety: Containers should be labeled with the compound's name, chemical formula, and hazard information. Safety data sheets (SDS) should be readily available to inform users about proper handling and potential risks.
![Article illustration]() Reference
Reference
[1] Wu Y, Xu P, Li L. Synthesis of alumina with coarse particle by precipitating aluminum ammonium sulfate solution with ammonia[J]. Advanced Powder Technology, 2016, 27(1): 124-129.
[2] Lin S H, Lo M C. Synthesis of aluminum ammonium sulfate from waste aluminum processing solution by crystallization[J]. Journal of hazardous materials, 1998, 63(2-3): 211-222.
);See also
Lastest Price from Aluminum ammonium sulfate manufacturers

US $3.60/kg2024-09-19
- CAS:
- 7784-25-0
- Min. Order:
- 1kg
- Purity:
- ≥99%
- Supply Ability:
- 3000tons/month

US $6.00/kg2024-09-19
- CAS:
- 7784-25-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month