3-Mercaptopropionic acid: Biosynthesis and Determination
General Description
The biosynthesis of 3-mercaptopropionic acid in Methanocaldococcus jannaschii involves unique enzymatic reactions leading from 2-hydroxy-4-mercaptobutyric acid to 3-mercaptopropionic acid. The pathway includes the conversion of malate semialdehyde to HMBA and further oxidation of HMBA to 4-mercapto-2-oxobutyric acid by L-sulfolactate dehydrogenase. Finally, HMBA is transformed into 3-mercaptopropionic acid. Understanding this pathway sheds light on sulfur metabolism in extreme environments and the ecological roles of 3-mercaptopropionic acid. A sensitive method based on pre-column derivatization and HPLC has been developed for its determination, showcasing excellent linearity, precision, and stability for accurate quantification even at low concentrations in environmental samples.

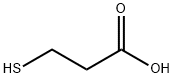
Figure 1. 3-Mercaptopropionic acid
Biosynthesis
Discovery and Initial Intermediates
3-Mercaptopropionic acid is a significant sulfur-containing metabolite found in marine environments, often resulting from both biotic and abiotic transformations. Its biosynthesis in Methanocaldococcus jannaschii, a hyperthermophilic methanogen, involves a unique pathway with several interesting intermediates and enzymatic reactions. The discovery of 3-Mercaptopropionic acid biosynthesis in M. jannaschii occurred during a non-targeted analysis aimed at identifying thiol-containing compounds. The research revealed 3-Mercaptopropionic acid alongside two previously unreported metabolites, 2-hydroxy-4-mercaptobutyric acid (HMBA) and 4-mercapto-2-oxobutyric acid (MOB), which are critical in the biosynthetic pathway of 3-Mercaptopropionic acid.
Enzymatic Reactions in the Pathway
The initial step in this pathway involves the biosynthesis of HMBA. This process is theorized to occur through a reaction involving malate semialdehyde and hydrogen sulfide. This reaction mechanism is similar to those found in the biosynthesis of other thiol-containing compounds in methanogens such as coenzyme M, coenzyme B, and homocysteine. Here, an aldehyde group is converted into a thiol group, forming the HMBA intermediate. Following the formation of HMBA, the enzyme L-sulfolactate dehydrogenase, encoded by the MJ1425 gene in M. jannaschii, catalyzes the NAD-dependent oxidation of HMBA to produce MOB. This enzymatic conversion is crucial as it links the earlier biosynthesis steps to the formation of 3-Mercaptopropionic acid.
Ecological and Evolutionary Implications
The final step in the biosynthesis of 3-mercaptopropionic acid involves the conversion of HMBA into 3-Mercaptopropionic acid. This transformation is facilitated within the cellular environment of M. jannaschii, indicating a specialized metabolic route that allows this organism to produce MPA efficiently. Understanding the biosynthesis of 3-mercaptopropionic acid in Methanocaldococcus jannaschii is not only vital for comprehending sulfur metabolism in extreme environments but also indicates the ecological roles of 3-Mercaptopropionic acid. The pathway suggests that 3-Mercaptopropionic acid likely serves critical functions for M. jannaschii, possibly related to cellular defense mechanisms or metabolic regulation in high-sulfur, high-temperature habitats. This biosynthetic pathway underscores the biochemical diversity and adaptability of M. jannaschii, reflecting its evolutionary optimization for survival in harsh oceanic environments. 1
Determination
Importance and Challenges
The determination of 3-mercaptopropionic acid is crucial for understanding organic sulfur metabolism in natural environments. As an important thiol intermediate generated during the degradation of sulfur-containing amino acids and the demethylation of dimethylsulfoniopropionate (DMSP), 3-mercaptopropionic acid plays a significant role in diverting sulfur away from dimethyl sulfide (DMS), a compound with climatic implications. Developing a sensitive method for the determination of 3-mercaptopropionic acid is challenging due to its high reactivity. However, a method has been devised based on pre-column derivatization with monobromobimane followed by analysis using high-performance liquid chromatography (HPLC) with fluorescence detection. This method has shown promise for accurately quantifying 3-mercaptopropionic acid in various matrices. During method development, 3-mercaptopropionic acid standards were utilized to optimize conditions for both low and high concentrations.
Method Optimization and Validation
Buffer selection for the derivatization step was crucial, with Tris-HCl buffer proving to be more effective in separating thiol derivatives and providing better peak shapes for 3-mercaptopropionic acid. The method demonstrated a detection limit of 4.3nmolL(-1) with a 10μL sample injection, ensuring sensitivity even at low concentrations. Furthermore, the method's reliability was confirmed through rigorous validation, including assessments of linearity, precision, and stability of derivatized samples. The method exhibited excellent linearity (r>0.99) and repeatability (% CV of 2.68-7.01% intra-day and 4.86-12.5% inter-day), indicating its suitability for quantitative analysis. One notable advantage of this method is the stability of derivatized samples when stored at 4°C, with minimal loss observed even after more than a year of storage. This enhanced stability allows for more flexible sample handling and analysis timelines. The effectiveness of the optimized methodology was further demonstrated through its successful application in measuring 3-mercaptopropionic acid concentrations and production rates in intertidal estuarine sediment slurries. The method proved efficient, offering straightforward sample derivatization and simple analysis of stable 3-MPA derivatives, thus facilitating accurate determination of 3-mercaptopropionic acid in environmental samples. 2
Reference
1. Allen KD, White RH. Occurrence and biosynthesis of 3-mercaptopropionic acid in Methanocaldococcus jannaschii. FEMS Microbiol Lett. 2016; 363(19): fnw217.
2. Salgado P, Visnevschi-Necrasov T, Kiene RP, et al. Determination of 3-mercaptopropionic acid by HPLC: A sensitive method for environmental applications. J Chromatogr B Analyt Technol Biomed Life Sci. 2015; 992: 103-108.
);Related articles And Qustion
Lastest Price from 3-Mercaptopropionic acid manufacturers

US $100.00-75.00/kg2024-09-19
- CAS:
- 107-96-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000

US $10.00/kg2024-09-19
- CAS:
- 107-96-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 10 ton