1-Benzyl-4-Piperidone: Applications in Medicinal Chemistry and its Preparation Methods
General Description
1-Benzyl-4-piperidone is a notable compound in medicinal chemistry, recognized for its therapeutic applications, particularly in developing menin inhibitors, which target specific protein-protein interactions linked to mixed lineage leukemia. These inhibitors play a crucial role in treating acute leukemia characterized by mixed lineage leukemia fusions by disrupting the menin-mixed lineage leukemia interaction, thereby potentially halting leukemia cell progression. 1-Benzyl-4-piperidone has been instrumental in the design of highly potent derivatives like M-89, which show remarkable efficacy and selectivity against mixed lineage leukemia fusion-carrying leukemia cell lines. Moreover, 1-Benzyl-4-piperidone can be synthesized through various methods, including a novel one-pot reaction that enhances yield and purity, making it suitable for industrial-scale production and further therapeutic applications.

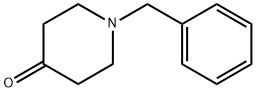
Figure 1. 1-Benzyl-4-piperidone
Applications in Medicinal Chemistry
1-Benzyl-4-piperidone has emerged as a significant compound in medicinal chemistry due to its promising therapeutic applications, particularly in targeting specific protein-protein interactions associated with various diseases. One of the notable applications of 1-Benzyl-4-piperidone involves its use as a key intermediate in the synthesis of menin inhibitors. These inhibitors are crucial for developing therapies aimed at treating acute leukemia characterized by the presence of mixed lineage leukemia fusions. By inhibiting the menin-mixed lineage leukemia protein-protein interaction, 1-Benzyl-4-piperidone-based compounds can potentially halt the progression of leukemia cells, offering a novel strategy to combat this aggressive form of cancer. 1
Development of Therapeutics Derived from 1-Benzyl-4-piperidone
The utility of 1-Benzyl-4-piperidone extends beyond its role as an intermediate. Its structural attributes allow for the design of highly potent inhibitors, exemplified by compounds like M-89, which have demonstrated remarkable efficacy in binding to menin at exceptionally low concentrations. The application of 1-Benzyl-4-piperidone in the development of these inhibitors shows a preferential activity against cancer cell lines harboring mixed lineage leukemia fusion while maintaining low toxicity towards other leukemia cell lines. This specificity is critical for minimizing side effects and improving treatment outcomes. As research continues, the derivative compounds of 1-Benzyl-4-piperidone hold promise in forming a new class of therapeutics designed specifically for targeting mixed lineage leukemia-related pathologies, highlighting the compound's important role in the future of cancer treatment strategies. 1
Preparation Methods
Overview
The preparation of 1-Benzyl-4-piperidone can be conducted through several synthetic routes. One well-known method involves using 4-piperidone or its hydrochloride salt, which reacts with alkylbenzyl halides via nucleophilic substitution. While this route may yield suitable amounts of 1-Benzyl-4-piperidone, the reactants are often expensive and difficult to store, making the process less viable for industrial applications. Another documented route involves the reduction of 1-benzoyl-4-piperidone, utilizing triethylsilane as a reducing agent. However, this approach poses safety risks because triethylsilane is highly flammable and can release hydrogen gas when improperly handled. The need for a high-performance setup further complicates scaling the process. Despite these methods facilitating the production of 1-Benzyl-4-piperidone, they often suffer from low yields and inadequate purity, presenting challenges for mass production. 2
An Efficient Preparation Method
A newly proposed technique for synthesizing 1-Benzyl-4-piperidone involves a one-pot reaction. Initially, benzylamine and an organic solvent are mixed in a reactor, followed by the addition of an appropriate amount of acrylate, in a molar ratio between 2.6 and 5. After stirring for one hour, the mixture is heated to a range of 50 to 60 degrees Celsius and maintained at that temperature for an additional 9 to 24 hours. This acrylate excess reduces the formation of mono-ester byproducts, thereby enhancing the purity of 1-Benzyl-4-piperidone. Following the reaction, unreacted acrylate and solvent are recovered, typically via distillation at atmospheric pressure for better material recovery. The process continues by introducing organic bases and maintaining the system at 50 to 85 degrees Celsius for 9 to 16 hours, ultimately leading to the collection of high-purity 1-Benzyl-4-piperidone suitable for industrial production with optimal yield and simplicity. 2
Reference
1. Aguilar A, Zheng K, Xu T, et al. Structure-Based Discovery of M-89 as a Highly Potent Inhibitor of the Menin-Mixed Lineage Leukemia (Menin-MLL) Protein-Protein Interaction. J Med Chem. 2019; 62(13): 6015-6034.
2. Zhao AM, Cao Z, Xu BQ. Preparation of N-benzyl-4-piperidone. 2023; Patent Number: CN116924967.
);Lastest Price from 1-Benzyl-4-piperidone manufacturers

US $10.00/kg2024-09-19
- CAS:
- 3612-20-2
- Min. Order:
- 1kg
- Purity:
- 99.9%
- Supply Ability:
- 10000

US $60.00/kg2024-09-19
- CAS:
- 3612-20-2
- Min. Order:
- 1kg
- Purity:
- 99.9%
- Supply Ability:
- 2000kg