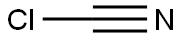Cyanide poisoning was first reported with the effects of extract
of bitter almonds; then cyanide was identified and isolated
from cherry laurel. Cyanogen chloride was first prepared in
1787 by the action of chlorine upon hydrocyanic acid (aka
prussic acid) and was called ‘oxidized prussic acid.’ The correct
formula for cyanogen chloride was first established in 1815.
Cyanogen chloride was used in World War I in 1916.
Cyanogen chloride is a colorless gas or liquid
(below 55℃
F/13℃
) with a pungent, irritating odor. Shipped
as a liquefied gas. A solid below—6℃
.
Cyanogen chloride (CK) is a very volatile compound, but is less a fire or explosive hazard than hydrogen cyanide and therefore logistically speaking less problematic. (Industry has found cyanogen chloride the preferred reactant in processes to make synthetic rubber). Reportedly, France combined hydrocyanic acid with cyanogen chloride in World War I ("manguinite"). The use of cyanogen chloride in this mixture was intended as an irritant to make soldiers remove their masks, exposing themselves to these very toxic gases. Cyanogen chloride was also combined with arsenic trichloride later on in the war. Like hydrocyanic acid, cyanogen chloride tends to spontaneously polymerize and therefore was combined with stabilizers (sodium pyrophosphate) for longer shelf life.
Cyanogen chloride is used in organic synthesis and as a military poisonous gas. Several benzene derivatives react with chloramine or with hypochlorous acid in the presence of ammonium ion to form cyanogen chloride (Maeda et al. 1987).
In chemical synthesis. Military poison gas.
Organic synthesis; poison gas used by
military
Cyanogen chloride is produced by the action of chlorine on
moist sodium cyanide suspended in carbon tetrachloride
and kept cooled to -3°C, followed by distillation.
A colorless gas or liquid with a strong acrid/pungent odor. Boils at 60°F. Liquid density 10.0 lb / gal. Shipped as a liquid confined under its own vapor pressure. A highly toxic lachrymator. Has been used as a tear gas. Vapor is heavier than air. Prolonged exposure of the container to fire or intense heat may cause violent rupturing and rocketing.
Soluble in water. Very slow reaction with water to form hydrogen cyanide.
CYANOGEN CHLORIDE may trimerize violently to form cyanuric chloride, catalyzed by hydrogen chloride or ammonium chloride. Reacts exothermically with alkenes and alkynes. Benzene and cyanogen halides yield HCl as a byproduct (Hagedorn, F. H. Gelbke, and Federal Republic of Germany. 2002. Nitriles. In Ullman Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA.).
Cyanogen chloride becomes volatile as temperatures increase, and the DOT lists it as a 2.3 poison gas. The NFPA 704 designation for CK is estimated to be health 4, flammability 0,reactivity 2, and special ?0. Cyanogen chloride vapors are highly toxic. It has a four-digit UN identification number of 1589 (inhibited). Treatment for either AC or CK poisoning is to follow the treatment protocols for airway, breathing, and circulation (ABCs) and administer oxygen to assist breathing. Instructions for administration and dosage should be based on local protocols and with the advice of a physician. Sodium nitrate is administered to produce methemoglobin, thus seizing the cyanide on the methemoglobin. The sodium thiosulfate combines with the confiscated cyanide to form thiocyanate, which is then excreted from the body.
VAPOR: POISONOUS IF INHALED OR IF SKIN IS EXPOSED. Irritating to eyes. LIQUID: POISONOUS IF SWALLOWED. Will burn skin and eyes.
Cyanogen chloride is a highly poisonous compound and a severe irritant. In humans, exposure to 1 ppm for 10 minutes caused severe irritation of eyes and nose. Irritation of respiratory tract is followed by hemorrhage of the bronchi and trachea, as well as pulmonary edema.
The toxicity of cyanogen chloride is attributed to its relatively easy decomposition to cyanide ion in an aqueous medium. The cyanide attacks the cells in the body and interferes with the cellular metabolism. Tests on rats indicated that exposure to cyanogen chloride caused lacrimation and chronic pulmonary edema and somnolence. At a high concentration of 300 ppm, death occurred to the test animals. Inhalation of 48 ppm for 30 minutes was fatal to humans. In animals, subcutaneous intakes of 5 and 15 mg/kg were lethal to both dogs and rabbits.
Chronic exposure to cyanogen chloride can cause conjunctivitis and edema of the eyelid.
Not flammable. POISONOUS GASES ARE PRODUCED WHEN HEATED IN FIRE. Overheated containers can explode.
Poison by ingestion,
subcutaneous, and possibly other routes.
Toxic by inhalation. Human systemic effects
by inhalation: lachrymation, conjunctiva
irritation, and chronic pulmonary edema or
congestion. A primary irritant. A severe
human eye irritant. An insecticide.
Flammable when exposed to heat or flame.
When heated to decomposition or on
contact with water or steam, it will react to
produce highly toxic and corrosive fumes of
Cl-, CN-, and NOx. See also other cyanogen
entries, CYANIDE, and CHLORIDES.
Cyanaogen chloride is used as a fumi-
gant, metal cleaner; in ore refining; production of synthetic
rubber and in chemical synthesis. CK is used as a military
poison gas (blood agent). It forms cyanide in the body.
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. Medical observation is recommended for 24-48 h after breathing overexposure, as pulmonary edema may be delayed. As first aid for pulmonaryedema, a doctor or authorized paramedic may consideradministering a corticosteroid spray. If frostbite hasoccurred, seek medical attention immediately; do NOT rubthe affected areas or flush them with water. In order toprevent further tissue damage, do NOT attempt to removefrozen clothing from frostbitten areas. If frostbite has NOToccurred, immediately and thoroughly wash contaminatedskin with soap and water.
Use amyl nitrate capsules if symptoms develop. All areaemployees should be trained regularly in emergency measures for cyanide poisoning and in CPR. A cyanide antidotekit should be kept in the immediate work area and must berapidly available. Kit ingredients should be replaced every1-2 years to ensure freshness. Persons trained in the use ofthis kit, oxygen use, and CPR must be quickly available.
The primary effect of cyanide poisoning results from the inhibition
of the metal-containing enzymes, specifically, cytochrome
oxidase a3 (containing iron) within the mitochondria.
Cyanide poisons the mitochondrial electron transport chain
within cells and renders the body unable to derive energy (ATP)
from oxygen, therefore causing rapid death. Other mechanisms
include pulmonary arteriolar and/or coronary vasoconstriction
that results in cardiogenic shock and pulmonary edema.
Cyanide can also directly stimulate chemoreceptors in the aorta
and carotid artery, causing hyperpnea.
Corrosive, Toxic gas. Color Code—Yellow Stripe:Reactivity Hazard; Store separately in an area isolated fromflammables, combustibles, or other yellow-coded materials.Prior to working with this chemical you should be trainedon its proper handling and storage. Store in tightly closedcontainers in a cool, well-ventilated area. Provide ventilation along the floor as the vapors are heavier than air.Procedures for the handling, use, and storage of cylindersshould be in compliance with OSHA 1910.101 and1910.169, as with the recommendations of the CompressedGas Association.
UN1589 Cyanogen chloride, stabilized, Hazard
Class: 2.3; Labels: 2.3-Poisonous gas, 8-Corrosive material
Inhalation Hazard Zone A. Cylinders must be transported
in a secure upright position, in a well-ventilated truck.
Protect cylinder and labels from physical damage. The
owner of the compressed gas cylinder is the only entity
allowed by federal law (49CFR) to transport and refill
them. It is a violation of transportation regulations to refill
compressed gas cylinders without the express written per-
mission of the owner. Military driver shall be given full
and complete information regarding shipment and condi-
tions in case of emergency. AR 50-6 deals specifically with
the shipment of chemical agents. Shipments of agent will
be escorted in accordance with AR 740-32.
At room temperature, cyanogen chloride is a colorless gas with
a pungent, biting odor that has been described as ‘pepper-like.’
Cyanogen chloride is soluble in both water (6.00E + 04 mg l-1)
and most organic solvents (e.g., chloroform, ethanol, or
benzene); however, such mixtures often are unstable.
Based on the high volatility and rapid hydrolysis of cyanogen
chloride, its adsorption to soil and sediment is not an important
environmental fate process. Estimated vapor pressure of
1230 mmHg suggests that cyanogen chloride will volatilize
rapidly from water surfaces and it is expected to volatilize from
dry soil surfaces.
CK is incompatible with; or, may react
with most basic and acidic solvents. CK reacts slowly with water or water vapor forming toxic hydrogen cyanide and
hydrogen chloride. Cyanogen chloride may polymerize vio-
lently if contaminated with chlorine. CK is unstable; it may
be stabilized (i.e., inhibited) to prevent polymerization. In
crude form CK trimerizes violently if catalyzed by traces
of hydrogen chloride or ammonium chloride. Contact with
alcohols, acids, acid salts; amines, strong alkalis; olefins,
and strong oxidizers may cause fire and explosion. Heat
causes decomposition producing toxic and corrosive fumes
of hydrogen cyanide, hydrochloric acid, nitrogen oxides.
Reacts slowly with water or water vapor, forming hydrogen
chloride. Attacks copper, brass, and bronze in the presence
of moisture.
Return refillable compressed
gas cylinders to supplier. React with strong calcium hypo-
chlorite solution for 24 hours, then flush to sewer with
large volumes of water.