| Uses |
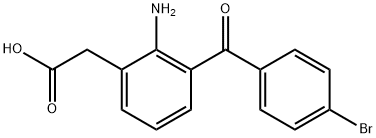
Bromfenac (Xibrom, ISTA Pharmaceuticals, Irvine, USA; Bronuck, Senju Pharmaceutical, Osaka, Japan) is indicated for the treatment of postoperative inflammation and the reduction of ocular pain in patients after undergoing cataract extraction. For this task, one drop of Xibrom may be applied to the affected eye twice daily beginning 24 hours after cataract surgery and continuing for the first 2 weeks of the postoperative period. The clinical safety and efficacy of bromfenac have been extensively studied in diverse comparative investigations, including the treatment of external or anterior ocular inflammatory diseases, allergic conjunctivitis, scleritis, and postoperative inflammation.The results of two phase III multicenter, randomized double-masked placebo-controlled clinical trials showed that bromfenac ophthalmic solution 0.09% was effective in the rapid resolution of ocular pain after cataract surgery, and there was a statistically significant difference between the bromfenac and placebo groups demonstrated in these phase III clinical trials.
|