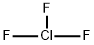Chemical Properties
Chlorine trifluoride is a greenish yellow,
almost colorless, liquid (below 12C/53F), or colorless gas
with a sweet, irritating odor. Shipped as a liquefied compressed gas.
Chemical Properties
yellowish gas or liquid
Uses
Fluorinating agent; in nuclear reactor fuel processing; incendiary; igniter and propellant for rockets; pyrolysis inhibitor for fluorocarbon polymers.
General Description
A colorless gas or green liquid with a pungent odor. Boils at 53°F. CHLORINE TRIFLUORIDE(7790-91-2) reacts with water to form chlorine and hydrofluoric acid with release of heat. Contact with organic materials may result in spontaneous ignition. CHLORINE TRIFLUORIDE(7790-91-2) is corrosive to metals and tissue. Prolonged exposure to low concentrations or short term exposure to high concentrations may result in adverse health effects. Under prolonged exposure to fire or intense heat the container may violently rupture and rocket.
Reactivity Profile
CHLORINE TRIFLUORIDE is a low-boiling liquid (b.p. 12° C), in gaseous state irritating and toxic. A highly reactive oxidant reagent, spontaneously flammable, used as a rocket propellant. Incompatible with fuels, nitro compounds. Interaction with water is violent and may be explosive, even with ice [Sidgwick, 1950, p. 1156]. Immediate explosive reaction with hydrocarbons or halocarbons even at-70° C [Brower, K. R., J. Fluorine Chem., 1986, 31, p. 333]. Solution with carbon tetrachloride capable of detonation, solutions with nitroaryl compounds (TNT, hexanitrobiphenyl) or highly chlorinated compounds are extremely shock-sensitive. Violent, sometimes explosive reaction with hydrogen containing materials, e.g., acetic acid, ammonia, benzene, ether, coal gas, hydrogen, hydrogen sulfide, methane, or fluoroamino compounds. Ignition with fibrous materials (cotton, paper, wood). [Mellor, 1956, vol. 2, suppl. 1, p. 155]. Explosive gaseous products (chlorodifluoroamine) formed with ammonium fluoride or ammonium hydrogen fluoride [Gardner, D. M. et al., Inorg., Chem., 1963, 2, p. 413]. Ignition on contact with iodine, boron-containing materials (boron powder, tetraboron carbide, boron-aluminum), fibrous or finely divided refractory materials (asbestos, glass, wool, sand, tungsten carbide). Violent reaction with mineral acids (nitric acid, sulfuric acid), chromium trioxide, ruthenium metal, selenium tetrafluoride. [Bretherick, 5th ed., 1995, p. 1235]. Chlorine trifluoride is a hypergolic oxidizer and contact with a number of metals and their oxides (aluminum, antimony, arsenic, calcium, copper, iridium, iron, lithium, lead, magnesium, molybdenum, osmium, potassium, rhodium, sodium, selenium, silver, tellurium, tin, tungsten, zinc), nonmetals (phosphorus, silicon, sulfur), salts (mercury iodide, potassium iodide, silver, nitrate, potassium carbonate) will result in a violent reaction often followed by ignition [Mellor, 1956, vol. 2, suppl. 1, p. 155; Sidgwick, 1950, p. 1156].
Air & Water Reactions
A violent reaction occurs with water or ice generating acidic HF and chlorine, [Sidgwick, 1156(1950)]. The release of Chlorine Trifluoride to the atmosphere rapidly generates two toxic reaction products: HF and Chlorine Dioxide, [Lombardi, D.A. and M.D. Cheng 1996. "Modeling Accidental Releases of Chlorine Trifluoride to the Atmosphere," Paper No. 96-WP66B.02, presented at the 89th Annual Meeting of the Air and Waste Management Association, Nashville, Tennessee, June 23-26].
Hazard
Explodes in contact with organic materials or with water. Dangerous fire risk. A poison,
very toxic, corrosive to skin. Lung damage, eye,
and upper respiratory tract irritant. Questionable
carcinogen.
Health Hazard
Inhalation causes extreme irritation of respiratory tract; pulmonary edema may result. Vapors are very irritating to eyes and skin; liquid causes severe burns.
Potential Exposure
Chlorine trifluoride is used as a fluorinating agent. It may be used as an igniter and propellant in
rockets. It is used in nuclear fuel processing.
First aid
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water or
milk. Do not induce vomiting. Medical observation is
recommended for 24 to 48 hours after breathing overexposure, as pulmonary edema may be delayed. As first aid for
pulmonary edema, a doctor or authorized paramedic may
consider administering a drug or other inhalation therapy.
If frostbite has occurred, seek medical attention immediately; do NOT rub the affected areas or flush them with
water. In order to prevent further tissue damage, do NOT
attempt to remove frozen clothing from frostbitten areas. If
frostbite has NOT occurred, immediately and thoroughly
wash contaminated skin with soap and water.
Shipping
UN1749 Chlorine trifluoride, Hazard class: 2.3;
Labels: 2.3-Poisonous gas, 5.1-Oxidizer, 8-Corrosive material, Inhalation Hazard Zone B. Cylinders must be transported in a secure upright position, in a well-ventilated
truck. Protect cylinder and labels from physical damage.
The owner of the compressed gas cylinder is the only entity
allowed by federal law (49CFR) to transport and refill
them. It is a violation of transportation regulations to refill
compressed gas cylinders without the express written permission of the owner.
Incompatibilities
A powerful oxidizer. Keep away from
acids. Most combustible materials ignite spontaneously
on contact with chlorine trifluoride. Explodes on contact
with organic materials. The liquid can explode if mixed
with halocarbons or hydrocarbons. It reacts violently
with oxidizable materials, finely divided metals and
metal oxides; sand, glass, asbestos, silicon-containing
compounds. Emits highly toxic fumes on contact with
acids. Chlorine trifluoride decomposes above 220C,
forming Thermal decomposition products may include
hydrogen chloride and HF. Reacts violently with water,
forming chlorine gas and hydrofluoric acid. Reacts with
most forms of plastics, rubber, coatings, and resins;
except the highly fluorinated polymers, such as Teflon
and “K el-F.”
Description
Chlorine trifluoride is a greenish yellow,almost colorless, liquid (below 12℃/53°F) or colorless gaswith a sweet, irritating odor. Shipped as a liquefied compressed gas. Molecular weight=92.45; Boilingpoint=11.8℃; Freezing/Melting point=2 76.3℃; Vaporpressure=1.4 atm; Relative vapor density (air=1)=3.21.Hazard Identification (based on NFPA 704 M RatingSystem): Health 4, Flammability 0, Reactivity 3 (Oxidizer).Reacts with water.
Waste Disposal
Return refillable compressed
gas cylinders to supplier.
Physical properties
Colorless gas; sweetish but suffocating odor; density of the liquid 1.77 g/mL at 13°C; condenses to a greenish yellow liquid at 11.75°C; freezes to a white solid at -76.3°C; reacts violently with water.
Definition
ChEBI: Trifluorochlorine is a halohalide.
Purification Methods
Impurities include chloryl fluoride, chlorine dioxide and hydrogen fluoride. Passed it first through two U-tubes containing NaF to remove HF, then through a series of traps in which the liquid is fractionally distilled. It can be purified via the KF complex; KClF4, formed by adding excess ClF3 to solid KF in a stainless steel cylinder in a dry-box and shaking overnight. After pumping out the volatile materials, pure ClF3 is obtained by heating the bomb to 100-150o and condensing the evolved gas in a -196o trap [Schack et al. Chem Ind (London) 545 1967]. It attacks glass very vigorously. HIGHLY TOXIC.
Fire Hazard
Nonflammable gas; dangerously reactive.
Chlorine trifluoride reacts explosively with
water, forming hydrogen fluoride and chlo rine. It reacts violently with most elements
and common substances. Paper, cloth, wood,
glass, wool, charcoal, and graphite burst
into flame in contact with the liquid. The
vapors, even when diluted, can set fire to
organic compounds. Reactions with most metals are vigorous to violent, often caus ing a fire. It catches fire when mixed with
phosphorus, arsenic, antimony, silicon, sul fur, selenium, tellurium, tungsten, osmium,
and rhodium (Mellor 1946, Suppl. 1956).
Among the alkali- and alkaline–earth metals,
reaction is violent with potassium at ordinary
temperatures, and with sodium, calcium, or
magnesium it reacts violently at elevated
temperatures. Violent reaction occurs with
oxides, sulfides, halides, and carbides of
metals, causing flames. Chlorine trifluoride
attacks sand, glass, and asbestos. Prolonged
contact can ignite glass. Explosive reactions
occur with many common gases, includ ing hydrogen, lower hydrocarbons, carbon
monoxide, ammonia, hydrogen sulfide, and
sulfur dioxide. Reactions with mineral acids
and alkalies are violent.
In case of a small fire involving chlorine
trifluoride, use a dry chemical or water
spray in large amounts (NFPA 1997). Allow
large fires to burn. Avoid contact of chlorine
trifluoride with the body or with protective
clothing.
storage
Chlorine trifluoride is stored and shippedin special steel cylinders. It is stored inmoisture-free, cool, and isolated areas sepa rated from other chemicals. The cylinders arekept upright, covered, and protected againstphysical damage.