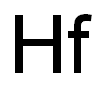Chemical Properties
Hafnium is a refractory metal which occurs in
nature in zirconium minerals.
Chemical Properties
solid
General Description
HAFNIUM POWDER, DRY(7440-58-6), is a grayish metallic colored powder. Dust from dry powder may be ignited by static electricity. The dry powder reacts with moisture to produce hydrogen, a flammable gas. The heat from this reaction may be sufficient to ignite the hydrogen. HAFNIUM POWDER, DRY(7440-58-6) does not appreciably react with large quantities of water.
Reactivity Profile
Metals, such as HAFNIUM METAL(reactivity similar to zirconium), are reducing agents and tend to react with oxidizing agents. Their reactivity is strongly influenced by their state of subdivision: in bulk they often resist chemical combination; in powdered form they may react very rapidly. Thus, as a bulk metal HAFNIUM POWDER, DRY is somewhat unreactive, but finely divided material may be pyrophoric. The metal reacts exothermically with compounds having active hydrogen atoms (such as acids and water) to form flammable hydrogen gas and caustic products. The reactions are less vigorous than the similar reactions of alkali metals, but the released heat can still ignite the released hydrogen. Materials in this group may react with azo/diazo compounds to form explosive products. These metals and the products of their corrosion by air and water can catalyze polymerization reactions in several classes of organic compounds; these polymerizations sometimes proceed rapidly or even explosively. Some metals in this group form explosive products with halogenated hydrocarbons.
Air & Water Reactions
Highly flammable. The dry powder reacts with moisture to produce hydrogen, a flammable gas. The heat from this reaction may be sufficient to ignite the hydrogen. HAFNIUM POWDER, DRY does not appreciably react with large quantities of water.
Health Hazard
Fire will produce irritating, corrosive and/or toxic gases. Inhalation of decomposition products may cause severe injury or death. Contact with substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution.
Potential Exposure
Hafnium metal has been used as a
control rod material in nuclear reactors. Thus, those
engaged in fabrication and machining of such rods may be
exposed.
Fire Hazard
Flammable/combustible material. May ignite on contact with moist air or moisture. May burn rapidly with flare-burning effect. Some react vigorously or explosively on contact with water. Some may decompose explosively when heated or involved in a fire. May re-ignite after fire is extinguished. Runoff may create fire or explosion hazard. Containers may explode when heated.
First aid
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, includ ing resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medi cal attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit.
Shipping
UN1326 Hafnium powder, wetted with not
<,25% water (a visible excess of water must be present)
(1) mechanically produced, particle size<53 μm; (2)
chemically produced, particle size<840 μm, Hazard Class:
4.1; Labels: 4.1-Flammable solid. UN2545 Hafnium pow der, dry, Hazard Class: 4.1; Labels: 4.1-Flammable solid.
UN1346 Hafnium powder, wetted with not less than 25%
water (a visible excess of water must be present)
(1) mechanically produced, particle size less than 53 μm;
(2) chemically produced, particle size less than 840 μm,
Hazard Class: 4.1; Labels: 4.1-Flammable solid.
Incompatibilities
Fine powder or dust may form explosive
mixture in air. The powder is highly flammable and a strong
reducing agent. The powder or dust reacts with moisture
forming flammable hydrogen gas; may spontaneously ignite
on contact with moist air; and at higher temperatures, with
nitrogen, phosphorous, oxygen, halogens, and sulfur; contact
with hot nitric acid; heat, shock, friction, strong oxidizers;
or ignition sources may cause explosions.
Description
De scription: Hafnium is a refractory metal which occurs innature in zirconium minerals. Molecular weight= 178.49;Specific gravity (H2O:1)= 13.31; Boiling point = 4602℃; .Freezing/Melting point = 2227℃; Vapor pressure= 1 X .10~4 mmHg at 20℃. Insoluble in water.
Waste Disposal
Recovery. Consider recycling,
otherwise, this chemical must be disposed of in compliance
with existing federal and local regulations.
Isotopes
There are 44 known isotopes for hafnium. Five are stable and one of the unstableisotopes has such a long half-life (Hf-174 with a 2.0×10+15 years) that it is includedas contributing 0.16% to the amount of hafnium found in the Earth’s crust. The percentagecontributions of the 5 stable isotopes to the element’s natural existence on Earth areas follows: Hf-176 = 5.26%, Hf-177 = 18.60%, Hf-178 = 27.28%, Hf-179 = 13.62%,and Hf-180 = 35.08%.
Origin of Name
Named after Hafnia, the Latin name for the city of Copenhagen, Denmark.
Occurrence
Hafnium is the 47th most abundant element on Earth. Thus, it is more abundant thaneither gold or silver. Because hafnium and zirconium are always found together in nature, bothmetals are refined and produced by the Kroll process. Pure samples of either hafnium or zirconiumare almost impossible to separate by the Kroll or other refining processes. Baddeleyite(ZrO2), a zirconium ore, and zircon (ZrSiO4) are treated with chlorine along with a carboncatalyst that produces a mixture of zirconium and hafnium tetrachlorides. These are reducedby using sodium or magnesium, resulting in the production of both metals. The molten metalsare separated by the process known as fractionation, which depends on their different meltingpoints and densities. As the mixture of the two metals cools during the fractionation process,the denser solidified hafnium sinks to the bottom of the vessel while the less dense zirconium(with a higher melting point than hafnium) floats on top.
Characteristics
As the first element in the third series of the transition elements, hafnium’s atomic number(72Hf ) follows the lanthanide series of rare-earths. The lanthanide series is separated out ofthe normal position of sequenced atomic numbers and is placed below the third series on theperiodic table (57La to 71Li). This rearrangement of the table allowed the positioning of elementsof the third series within groups more related to similar chemical and physical characteristics—for example, the triads of Ti, Zr, and Hf; V, Nb, and Ta; and Cu, Ag, and Au.
Definition
hafnium: Symbol Hf. A silvery lustrousmetallic transition element;a.n. 72; r.a.m. 178.49; r.d. 13.3; m.p.2227±20°C; b.p. 4602°C. The elementis found with zirconium and is extractedby formation of the chlorideand reduction by the Kroll process. Itis used in tungsten alloys in filamentsand electrodes and as a neutron absorber.The metal forms a passiveoxide layer in air. Most of its compoundsare hafnium(IV) complexes;less stable hafnium(III) complexesalso exist. The element was first reportedby Urbain in 1911, and its existencewas finally established by Dirk Coster (1889–1950) and Georgede Hevesey (1885–1966) in 1923.
Hazard
Although the metal hafnium is not harmful, its powder and dust are both toxic if inhaledand explosive even when wet.
Flammability and Explosibility
Highlyflammable
Industrial uses
Pure hafnium is a lustrous, silvery metal that is not so ductile nor so easily worked as zirconium; nevertheless, hafnium can be hot- and cold-rolled on the same equipment and with similar techniques as those used for zirconium. All zirconium chemicals and alloys may contain some hafnium, and hafnium metal usually contains about 2% zirconium.The metal has a closepacked hexagonal structure. The electric conductivity is about 6% that of copper. It has excellent resistance to a wide range of corrosive environments.
Because of the startling similarity in their chemical properties, zirconium and hafnium always occur together in nature. In their respective ability to absorb neutrons, however, they differ greatly, and this difference has led to their use in surprisingly different ways in nuclear reactors. Zirconium, with a low neutron-absorption cross section (0.18 barn), is highly desirable as a structural material in water-cooled nuclear reactor cores. Hafnium, on the other hand, because of its high neutron-absorption cross section (105 barns), can be used as a neutron-absorbing control material in the same nuclear reactor cores. Thus, the two elements, which occur together so intimately in nature that they are very difficult to separate, are used as individual and important but contrasting components in the cores of nuclear reactors.
storage
Color Code- -Yellow Stripe (dry powder is a strong reducing agent): Reactivity Hazard; Store separately in anarea isolated from flammables, combustibles, or other yellow coded materials. Prior to working with this chemical youshould be trained on its proper handling and storage. Store intightly closed containers in a cool, well-ventilated area.