69-72-7
| Name | Salicylic acid |
| CAS | 69-72-7 |
| EINECS(EC#) | 200-712-3 |
| Molecular Formula | C7H6O3 |
| MDL Number | MFCD00002439 |
| Molecular Weight | 138.12 |
| MOL File | 69-72-7.mol |
Synonyms
2-HYDROXYBENZOIC ACID
ACETYLSALICYLIC ACID IMPURITY C
ACETYLSALISYLIC ACID IMP C
ACIDUM SALICYLICUM
FEMA 3985
O-HYDROXYBENZOIC ACID
O-OXYBENZOIC ACID
RARECHEM AL BO 0194
RETARDER TSA
RETARDER W
SALICYCLIC ACID
SALICYLIC ACID
2-Carboxyphenol
2-Hydroxybenzenecarboxylic acid
Acido salicilico
acidoo-idrossibenzoico
acidosalicilico
Advanced pain relief callus removers
Advanced pain relief corn removers
Benzoic acid, o-hydroxy-
Chemical Properties
| Appearance | Salicylic acid is a white to tan crystalline solid; needles. |
| Melting point | 158-161 °C(lit.) |
| Boiling point | 211 °C(lit.) |
| density | 1.44 |
| vapor density | 4.8 (vs air) |
| vapor pressure | 1 mm Hg ( 114 °C) |
| FEMA | 3985 |
| refractive index | 1,565 |
| Fp | 157 °C |
| storage temp. | Store at RT. |
| solubility | ethanol: 1 M at 20 °C, clear, colorless |
| form | Solid |
| pka | 2.98(at 25℃) |
| color | White to off-white |
| Odor | at 100.00 %. faint phenolic nutty |
| PH | 2.4 (H2O)(saturated solution) |
| PH Range | Non0 uorescence (2.5) to dark blue 0 uorescence (4.0) |
| Stability: | Stable. Substances to be avoided include oxidizing agents, strong bases, iodine, fluorine. Combustible. Sensitive to light. |
| Odor Type | nutty |
| Water Solubility | 1.8 g/L (20 ºC) |
| Sensitive | Light Sensitive |
| Usage | Acetylsalicylic Acid Impurity B |
| λmax | 210nm, 234nm, 303nm |
| Detection Methods | HPLC,NMR |
| JECFA Number | 958 |
| Merck | 14,8332 |
| Sublimation | 70 ºC |
| BRN | 774890 |
| Major Application | Semiconductors, nanoparticles, photoresists, lubricating oils, UV absorbers, adhesive, leather, cleaner, hair dye, soaps, cosmetics, pain medication, analgesics, antibacterial agent, treatment of dandruff, hyperpigmented skin, tinea pedis, onychomycosis, osteoporosis, beriberi, fungicidal skin disease, autoimmune disease |
| InChIKey | YGSDEFSMJLZEOE-UHFFFAOYSA-N |
| LogP | 2.01 |
| Uses |
salicylic acid is a beta-hydroxy acid with keratolytic and antiinflammatory activity. It helps dissolve the top layer of stratum corneum cells, improving the look and feel of the skin. Salicylic acid is an effective ingredient in acne products and as such is widely used in acne soaps and lotions. Because it is lipid soluble, it can more easily reduce sebaceous follicle blockage by penetrating the pores and exfoliating the cellular buildup. It is antimicrobial, anti-septic, enhances the activity of preservatives, and can be used to adjust the pH of products. For the treatment of aging skin, it appears to help improve skin wrinkles, roughness, and tone. In addition, it is a useful ingredient for products formulated to treat psoriasis, callouses, corns, and warts-cases where there is a buildup of dead skin cells. When applied topically, it is reported to penetrate 3 to 4 mm into the epidermis. A small amount of salicylic acid can convert to copper salicylate, a powerful anti-inflammatory. used at high concentrations, salicylic acid may cause skin redness and rashes. This is a naturally occurring organic acid, related to aspirin. It is found in some plants, particularly the leaves of wintergreen, willow bark, and the bark of sweet birch. Salicylic acid is also synthetically manufactured.
|
| CAS DataBase Reference | 69-72-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzoic acid, 2-hydroxy-(69-72-7) |
| Storage Precautions | Light sensitive |
| EPA Substance Registry System | 69-72-7(EPA Substance) |
Safety Data
| Hazard Codes | Xn |
| Risk Statements |
R22:Harmful if swallowed.
R41:Risk of serious damage to eyes. R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Statements |
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S39:Wear eye/face protection . S37/39:Wear suitable gloves and eye/face protection . S36:Wear suitable protective clothing . |
| RIDADR | UN 1648 3 / PGII |
| WGK Germany | 1 |
| RTECS | VO0525000 |
| Autoignition Temperature | 500 °C |
| TSCA | Yes |
| HS Code | 29182100 |
| Safety Profile |
Poison by ingestion, intravenous, and intraperitoneal routes. Moderately toxic by subcutaneous route. An experimental teratogen. Human systemic effects by skin contact: ear tinnitus. Mutation data reported. A skin and severe eye irritant. Experimental reproductive effects. Incompatible with iron salts, spirit nitrous ether, lead acetate, iodine. Used in the manufacture of aspirin. When heated to decomposition it emits acrid smoke and irritating fumes.
|
| Hazardous Substances Data | 69-72-7(Hazardous Substances Data) |
| Toxicity |
LD50 i.v. in mice: 500 mg/kg (Sota)
|
Raw materials And Preparation Products
Raw materials
1of2
Hazard Information
General Description
Odorless white to light tan solid. Sinks and mixes slowly with water.
Reactivity Profile
SALICYLIC ACID(69-72-7) is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in SALICYLIC ACID(69-72-7) to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions.
Air & Water Reactions
Sublimes and forms vapor or dust that may explode [USCG, 1999].
Hazard
Respiratory alkalosis, hyperkalemia,
hyperthermia, dehydration, convulsions, shock, res-
piratory paralysis, respiratory acidosis, lesions and
death from respiratory collapse; fetotoxic.
Health Hazard
Inhalation of dust irritates nose and throat. Vomiting may occur spontaneously if large amounts are swallowed. Contact with eyes causes irritation, marked pain, and corneal injury which should heal. Prolonged or repeated skin contact may cause marked irritation or even a mild burn.
Potential Exposure
Used as a topical keratolytic agent; in
manufacture of aspirin, salicylates, resins, as a dyestuff
intermediate; prevulcanization inhibitor; analytical reagent;
fungicide, antiseptic, and food preservative.
First aid
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. Skin contact: remove
contaminated clothing and wash immediately with soap and
water. Seek medical attention immediately. If this chemical
has been inhaled, remove from exposure, begin rescue
breathing (using universal precautions, including resuscita-
tion mask) if breathing has stopped and CPR if heart action
has stopped. Transfer promptly to a medical facility. When
this chemical has been swallowed, get medical attention.
Give large quantities of water and induce vomiting. Do not
make an unconscious person vomit.
Incompatibilities
iron salts; lead acetate; iodine. Forms an
explosive mixture in air.
Description
Salicylic acid (from Latin salix, willow tree, from the bark of which the substance used to be obtained) is a mono hydroxy benzoic acid, a type of phenolic acid and a beta hydroxy acid. This colorless crystalline organic acid is widely used in organic synthesis and functions as a plant hormone. It is derived from the metabolism of salicin. In addition to being an important active metabolite of aspirin (acetylsalicylic acid), which acts in part as a prod rug to salicylic acid, it is probably best known for its use in anti-acne treatments. The salts and esters of salicylic acid are known as salicylates.
Chemical Properties
2-Hydroxybenzoic acid is odorless or has a slight phenolic odor with an acrid taste.
Chemical Properties
Also known as o-hydroxybenzoic acid,C6H4(OH)(COOH) is a white powder with an acrid taste that is stable in air but gradually discolored by light. Soluble in acetone, oil of turpentine, alcohol, ether,benzene; slightly soluble in water,and combustible. Derived by reacting a hot solution of sodium phenolate with carbon dioxide and acidifying the sodium salt thus formed. Used in the manufacture of aspirin and salicylates, resins, dyestuff intermediate, prevulcanization inhibitor, analytical reagent,and fungicide.
Chemical Properties
Salicylic acid has the formula C6H4(OH) COOH, where the OH group is ortho to the carboxyl group. It is also known as 2- hydroxybenzoic acid. It is poorly soluble in water (2 g / L at 20 °C). Aspirin (acetyl salicylic acid or ASA) can be prepared by the esterification of the phenolic hydroxyl group of salicylic acid with the acetyl group from acetic anhydride or acetyl chloride.
Chemical Properties
Salicylic acid is a white to tan crystalline
solid; needles.
Physical properties
Salicylic acid. Appearance: white crystalline powder. Solubility: Absolutely soluble
in ethanol, soluble in ether and chloroform, slightly soluble in water and anhydrous
ether. Stability: Stable at room temperature, discomposes into phenol and carbon
dioxide after rapidly heated. It’s partially acidic.
Aspirin. Appearance: white crystal and decomposes at 136–140? °C. Melting point: 136?°C.?Aspirin is the acetyl derivative of salicylic acid with weak acidity. Its acidity coefficient is 3.5 at 25?°C. Stability: Aspirin decomposes rapidly in ammonium acetate, alkali metal of acetate, carbonate, citrate or hydroxide solutions. There are two crystal forms of aspirin including crystal form I and II.
Aspirin. Appearance: white crystal and decomposes at 136–140? °C. Melting point: 136?°C.?Aspirin is the acetyl derivative of salicylic acid with weak acidity. Its acidity coefficient is 3.5 at 25?°C. Stability: Aspirin decomposes rapidly in ammonium acetate, alkali metal of acetate, carbonate, citrate or hydroxide solutions. There are two crystal forms of aspirin including crystal form I and II.
Occurrence
Unripe fruits and vegetables are natural sources of salicylic acid, particularly blackberries, blueberries, cantaloupes, dates, raisins, kiwi fruits, guavas, apricots, green pepper, olives, tomatoes, radish and chicory; also mushrooms. Some herbs and spices contain quite high amounts, although meat, poultry, fish, eggs and dairy products all have little to no salicylates. Of the legumes, seeds, nuts, and cereals, only almonds, water chestnuts and peanuts have significant amounts.
History
The Greek physician Hippocrates wrote in the 5th century BC about a bitter powder extracted from willow bark that could ease aches and pains and reduce fevers . This remedy was also mentioned in texts from ancient Sumer , Lebanon , and As syria .
The active extract of the bark, called salicin, after the Latin name for the white willow (Salix alba), was isolated and named by the German chemist Johann Andreas Buchner in 1826. Raffaele Piria, an Italian chemist was able to convert the substance into a sugar and a second component, which on oxidation becomes salicylic acid.
Salicylic acid was also isolated from the herb meadowsweet (Filipendula ulmaria, formerly classified as Spiraea ulmaria) by German researchers in 1839. While their extract was somewhat effective, it also caused digestive problems such as gastric irritation, bleeding, diarrhea, and even death when consumed in high doses.
The active extract of the bark, called salicin, after the Latin name for the white willow (Salix alba), was isolated and named by the German chemist Johann Andreas Buchner in 1826. Raffaele Piria, an Italian chemist was able to convert the substance into a sugar and a second component, which on oxidation becomes salicylic acid.
Salicylic acid was also isolated from the herb meadowsweet (Filipendula ulmaria, formerly classified as Spiraea ulmaria) by German researchers in 1839. While their extract was somewhat effective, it also caused digestive problems such as gastric irritation, bleeding, diarrhea, and even death when consumed in high doses.
Definition
A crystalline
aromatic carboxylic acid. It is used in medicines, as an antiseptic, and in the manufacture of azo dyes. Its ethanoyl (acetyl)
ester is aspirin. See aspirin; methyl salicylate.
Definition
ChEBI: A monohydroxybenzoic acid that is benzoic acid with a hydroxy group at the ortho position. It is obtained from the bark of the white willow and wintergreen leaves.
Indications
Salicylic acid is a β-hydroxy acid that penetrates into the sebaceous gland
and has comedolytic and anti-inflammatory properties. It can be used as an
adjunctive therapy and is found in cleansers, toners, masks, and peels.
Salicylic acid is keratolytic and at concentrations between 3% and 6% causes softening of the horny layers and shedding of scales. It produces this desquamation by solubilizing the intercellular cement and enhances the shedding of corneocytes by decreasing cell-to-cell cohesion. In concentrations >6%, it can be destructive to tissue. Application of large amounts of the higher concentration of salicylic acid can also result in systemic toxicity. Salicylic acid is used in the treatment of superficial fungal infections, acne, psoriasis, seborrheic dermatitis, warts, and other scaling dermatoses. When it is combined with sulfur, some believe that a synergistic keratolytic effect is produced. Common preparations include a 3% and 6% ointment with equal concentration of sulfur; 6% propylene glycol solution (Keralyt); 5% to 20% with equal parts of lactic acid in flexible collodion for warts (Duofilm, Occlusal); in a cream base at any concentration for keratolytic effects; as a 60% ointment for plantar warts; and in a 40% plaster on velvet cloth for the treatment of calluses and warts (40% salicylic acid plaster).
Salicylic acid is keratolytic and at concentrations between 3% and 6% causes softening of the horny layers and shedding of scales. It produces this desquamation by solubilizing the intercellular cement and enhances the shedding of corneocytes by decreasing cell-to-cell cohesion. In concentrations >6%, it can be destructive to tissue. Application of large amounts of the higher concentration of salicylic acid can also result in systemic toxicity. Salicylic acid is used in the treatment of superficial fungal infections, acne, psoriasis, seborrheic dermatitis, warts, and other scaling dermatoses. When it is combined with sulfur, some believe that a synergistic keratolytic effect is produced. Common preparations include a 3% and 6% ointment with equal concentration of sulfur; 6% propylene glycol solution (Keralyt); 5% to 20% with equal parts of lactic acid in flexible collodion for warts (Duofilm, Occlusal); in a cream base at any concentration for keratolytic effects; as a 60% ointment for plantar warts; and in a 40% plaster on velvet cloth for the treatment of calluses and warts (40% salicylic acid plaster).
Preparation
Prepared by heating sodium phenolate with carbon dioxide under pressure
Production Methods
Salicylic acid is biosynthesized from the amino acid phenylalanine. In Arabidopsis thaliana it can also be synthesized via a phenylalanine - independent pathway.
Sodium salicylate is commercially prepared by treating sodium phenolate ( the sodium salt of phenol ) with carbon dioxide at high pressure (100 atm ) and high temperature (390K) -a method known as the Kolbe-Schmitt reaction. Acidification of the product with sulfuric acid gives salicylic acid :
It can also be prepared by the hydrolysis of aspirin (acetylsalicylic acid) or methyl salicylate (oil of winter green) with a strong acid or base.
Sodium salicylate is commercially prepared by treating sodium phenolate ( the sodium salt of phenol ) with carbon dioxide at high pressure (100 atm ) and high temperature (390K) -a method known as the Kolbe-Schmitt reaction. Acidification of the product with sulfuric acid gives salicylic acid :
It can also be prepared by the hydrolysis of aspirin (acetylsalicylic acid) or methyl salicylate (oil of winter green) with a strong acid or base.
Production Methods
Salicylic acid may be obtained (1) from oil of wintergreen, which contains methyl salicylate, or (2) by heating dry sodium phenate C6H5ONa plus carbon dioxide under pressure at 130 °C (266 °F) and recovering from the resulting sodium salicylate by adding dilute sulfuric acid.
Brand name
Advanced Pain Relief Callus Removers (Schering-Plough HealthCare); Advanced Pain Relief Corn Removers (Schering-Plough HealthCare); Clear Away Wart Remover (Schering-Plough HealthCare); Compound W (Whitehall-Robins); Dr. Scholl’s Callus Removers (Schering-Plough HealthCare); Dr. Scholl’s Corn Removers (Schering-Plough HealthCare); Dr. Scholl’s Wart Remover Kit (Schering-Plough HealthCare); Duofilm Wart Remover (Schering-Plough HealthCare); Duoplant (Stiefel); Freezone (Whitehall-Robins); Ionil (Galderma); Ionil-Plus (Galderma); Salicylic Acid Soap (Stiefel); Saligel (Stiefel); Stri-Dex (Sterling Health U.S.A.).
Synthesis Reference(s)
The Journal of Organic Chemistry, 27, p. 3551, 1962 DOI: 10.1021/jo01057a035
Tetrahedron Letters, 37, p. 153, 1996 DOI: 10.1016/0040-4039(95)02120-5
Tetrahedron Letters, 37, p. 153, 1996 DOI: 10.1016/0040-4039(95)02120-5
Biochem/physiol Actions
Salicylic acid (SA) plays a major role in the growth and development of plants. It also plays a role in endogenous signaling for plant disease resistance. SA is also involved in mediating thermogenesis and symptom development.
Mechanism of action
Salicylic acid has been shown to work through several different pathways. It produces its anti - inflammatory effects via suppressing the activity of cyclo oxygenase (COX), an enzyme which is responsible for the production of pro - inflammatory mediators such as the prostaglandins. Notably, it does this not by direct inhibition of COX, unlike most other non-steroidal anti-inflammatory drugs (NSAIDs), but instead by suppression of the expression of the enzyme (via a yet-un elucidated mechanism) . Salicylic acid has also been shown to activate adenosine monophosphate-activated protein kinase (AMPK), and it is thought that this action may play a role in the anticancer effects of the compound and its prod rugs aspirin and salsalate. In addition, the anti diabetic effects of salicylic acid are likely mediated by AMPK activation primarily through allosteric conformational change that increases levels of phosphorylation. Salicylic acid also uncouples oxidative phosphorylation which leads to increased ADP:ATP and AMP:ATP ratios in the cell. Consequently, salicylic acid may alter AMPK activity and subsequently exert its anti-diabetic properties through altered energy status of the cell. Even in AMPK knock - out mice, however, there is an anti-diabetic effect demonstrating that there is at least one additional, yet - unidentified action of the compound.
Pharmacology
Aspirin is a nonsteroidal anti-inflammatory drug (NSAID). The main pharmacological effect is to inhibit prostaglandin metabolism and thromboxane synthesis by
inhibiting prostaglandin metabolism-required cyclooxygenase, via irreversible acetylation of 530 serine residues in the hydroxyl of COX-1 polypeptide chain,
which results in COX-1 inactivation, blocks the conversion of arachidonic acid into
thromboxane A2 pathway and then inhibits the platelet aggregation.
Prostaglandin is a hormone produced locally in the body. It can pass the pain to the brain, regulate body temperature in the hypothalamus and cause inflammation. Inhibition of prostaglandin synthesis can have antipyretic, analgesic, antiinflammatory and antirheumatic effects. The adverse effects of aspirin are mainly gastrointestinal symptoms such as nausea, vomiting, upper abdominal discomfort or pain. It can also cause allergic reactions, cardiotoxicity, liver and kidney damage and Wright’s syndrome. In addition, high doses of aspirin can cause salicylic acid reactions such as headache, dizziness, tinnitus, hearing loss and other central nervous system symptoms.
Prostaglandin is a hormone produced locally in the body. It can pass the pain to the brain, regulate body temperature in the hypothalamus and cause inflammation. Inhibition of prostaglandin synthesis can have antipyretic, analgesic, antiinflammatory and antirheumatic effects. The adverse effects of aspirin are mainly gastrointestinal symptoms such as nausea, vomiting, upper abdominal discomfort or pain. It can also cause allergic reactions, cardiotoxicity, liver and kidney damage and Wright’s syndrome. In addition, high doses of aspirin can cause salicylic acid reactions such as headache, dizziness, tinnitus, hearing loss and other central nervous system symptoms.
Clinical Use
The clinical application of aspirin varies with the therapeutic dose. Low-dose aspirin (75–300?mg/day) has antiplatelet aggregation effect and can be used to prevent
and treat the coronary and cerebrovascular thrombosis and other postoperative
thrombosis. The middle dose of aspirin (0.5–3? g/day) has antipyretic analgesic
effects, so it is commonly used in the treatment of fever, headache, toothache, neuralgia, muscle pain and menstrual pain. High doses of aspirin (more than 4?g/day)
have anti-inflammatory and antirheumatic effects for the treatment of acute rheumatic fever and rheumatoid arthritis. In addition, aspirin is used for the treatment of
skin and mucous membrane lymphadenopathy (Kawasaki disease) in paediatric.
Side effects
Salicylic acid's side
effects include erythema and scaling.
Veterinary Drugs and Treatments
Often combined with sulfur, salicylic acid shampoos are often employed to treat patients with seborrheic disorders (seborrhea sicca and
oleosa) exhibiting mild to moderate scaling, with mild waxy and keratinous debris. In higher concentrations, topicals such as Kerasolv?
Gel (6.6% salicylic acid) can be used to remove localized excessive tissues associated with hyperkeratotic disorders, such as calluses and
idiopathic thickening of the planum nasale and footpads.
Salicylic acid has mildly antipruritic, antibacterial (bacteriostatic), keratoplastic and keratolytic actions. Lower concentrations are primarily keratoplastic and higher concentrations, keratolytic. Salicylic acid lowers skin pH, increases corneocyte hydration and dissolves the intercellular binder between corneocytes. Salicylic acid and sulfur are thought to be synergistic in their keratolytic actions.
Salicylic acid has mildly antipruritic, antibacterial (bacteriostatic), keratoplastic and keratolytic actions. Lower concentrations are primarily keratoplastic and higher concentrations, keratolytic. Salicylic acid lowers skin pH, increases corneocyte hydration and dissolves the intercellular binder between corneocytes. Salicylic acid and sulfur are thought to be synergistic in their keratolytic actions.
target
ROS | SOD
storage
4°C, protect from light
Purification Methods
It has been purified by steam distillation, by recrystallisation from H2O (solubility is 0.22% at room temperature and 6.7% at 100o), absolute MeOH, or cyclohexane and by sublimation in a vacuum at 76o. The acid chloride (needles) has m 19-19.5o, b 92o/15mm, the amide has m 133o (yellow needles from H2O), the O-acetyl derivative has m 135o (rapid heating and the liquid resolidifies at 118o), and the O-benzoyl derivative has m 132o (aqueous EtOH). [IR: Hales et al. J Chem Soc 3145 1954, Bergmann et al. J Chem Soc 2351 1950]. [Beilstein 10 IV 125.]
Plant hormone
Salicylic acid (SA) is a phenolic phytohormone and is found in plants with roles in plant growth and development, photosynthesis, transpiration, ion uptake and transport. SA also induces specific changes in leaf anatomy and chloroplast structure. SA is involved in endogenous signaling, mediating in plant defense against pathogens. It plays a role in the resistance to pathogens by inducing the production of pathogenesis-related proteins . It is involved in the systemic acquired resistance (SAR) in which a pathogenic attack on one part of the plant induces resistance in other parts. The signal can also move to nearby plants by salicylic acid being converted to the volatile ester, methyl salicylate.
Questions And Answer
-
description
Salicylic acid is an important organic synthetic raw material, widely used in medicine, pesticides, dyes, rubber, food and perfumes. In the pharmaceutical industry, the main drug productions of salicylic acid are sodium salicylate, wintergreen oil (methyl salicylate), aspirin (acetylsalicylic acid), salicylic acid amine, phenyl salicylate. In dye industry, it is used for production mordant pure yellow , direct yellow 3GN, direct yellow GR, direct brown 3GN, acid mordant brown G, acid mordant yellow GG, acid yellow dye complex. In pesticide production, salicylic acid is used for the synthesis of organic phosphorus pesticide Isocarbophos, intermediate isopropyl salicylate isofenphos methyl and Rodenticide warfarin, coumatetralyl intermediate 4-hydroxycoumarin. In rubber industry, it is used as anti scorching agent and production of ultraviolet absorbent and foaming agent. It is perfume material, used for the preparation of methyl salicylate, salicylic acid ethyl ester; food preservatives, its sodium Salt is mostly used, now a number of countries have been banned; methyl salicylate can be used as oral cleaning agents, such as toothpaste flavor.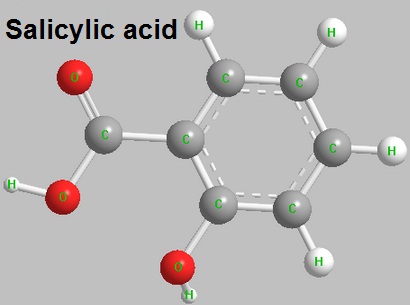
Figure1 The structure of salicylic acid
Salicylic acid has sterilization ability, 2.5% alcohol solution (called spiritus) is used as a topical medicine in the treatment of tinea manus and tinea pedis, also can be made into ointment. Sodium salicylate can be used for food preservative or preservatives, also can be used for the preparation of toothpaste, mouthwash. Salicylic acid has antipyretic and analgesic effects, can cure rheumatism and so on. It's all esters can be used as drug and spices. Methyl salicylate has pleasant aromas of holly, is used for the preparation of perfume and soap flavor. The methyl salicylate is coated on the skin, can penetrate into the muscle and let out of salicylic acid to release local pain, so it also can be used for the treatment of pain, contusion, sprain and other medication. Isoamyl salicylate has orchid aroma, benzyl salicylate has weak ester aroma, both can prepare perfume or soap flavor. The common name of phenyl salicylate is Salo, was hydrolyzed into phenol and salicylic acid in the intestine , is a kind of effective enteral preservatives. Another Kind drug related to salicylic acid is para aminosalicylic acid (PAS, see "Para amino salicylic acid), Its sodium and calcium salts are used as anti TB drugs for acute exudative pulmonary tuberculosis (TB) and mucosal tuberculosis, is a weak antimicrobial drug, the effect is only 2% of streptomycin, often combined with streptomycin and isoniazid , in order to enhance the curative effect. It can be acetylated to the aspirin.
The above information is Chemicalbook Hanya edited.; -
Chemical Properties
Salicylic acid is a white powder with an acrid taste that is stable in air but gradually discolored by light. Soluble in acetone, oil of turpentine, alcohol, ether,benzene; slightly soluble in water,and combustible. Derived by reacting a hot solution of sodium phenolate with carbon dioxide and acidifying the sodium salt thus formed. Used in the manufacture of aspirin and salicylates, resins, dyestuff intermediate, prevulcanization inhibitor, analytical reagent,and fungicide.; -
Benefits for Skin
Salicylic acid is a beta hydroxy acid that also sloughs dead cell buildup within the follicle, acts as a mild antibacterial, and has soothing properties. Salicylic acid is regarded to be less irritating than benzoyl peroxide and has less allergy potential, but it is also less aggressive in treating acne. It is often used for treating milder forms of acne. The concentration in OTC drugs is limited to 2 percent. Salicylic acid is also used as a performance ingredient exfoliant in smaller concentrations, without making a drug claim.; -
Application
Salicylic acid is widely used, eczema, psoriasis, salicylic acid can be used in acne, dandruff. The concentration of 3%~6% can be used to horny, higher than 6% of salicylic acid can damage to tissue . Below 40% of concentration is suitable for the treatment of thick cocoon, corns and warts. Salicylic acid can also be added in the treatment of acne and dandruff. Nowadays many famous cosmetic ingredients: in 1993, Clinique CLINIQUE first launched 1% salicylic acid in soft water cream, immediately became one of Clinique's most successful products; In 1998, SK-II crystal induced skin cream added 1.5%BHA ingredients to the original, and salicylic acid has effect of treatment pores and cutin like the egg peeling analogy that caused the market boom; open access Olay popular products activating cream also contains 1.5% BHA components. However, due to the high concentration of salicylic acid, it has a certain degree of damage, cosmetics containing salicylic acid concentration has generally been limited between 0.2%~1.5%, containing salicylic acid cosmetics shall be added to the note of warning signs to determine the safety of long-term use and children under 3 years of age also shall not be used.; -
Uses
Salicylic acid is an FDA approved skin care ingredient used for the topical treatment of acne, and it's the only beta hydroxy acid (BHA) used in skin care products. Perfect for oily skin, salicylic acid is best known for its ability to deep clean excess oil out of pores and reduce oil production moving forward. Because salicylic acid keeps pores clean and unclogged, it prevents future whiteheads and blackheads from developing. Salicylic acid also exfoliates dead skin, and its anti-inflammatory properties make it a prime ingredient for those with psoriasis. Salicylic acid naturally occurs in willow bark, sweet birch bark, and wintergreen leaves, but synthetic versions are also used in skin care products.; -
Methods of production
1. The phenol and sodium hydroxide react to produce phenol sodium, distillation and dehydration, CO2 carboxylation reaction to obtain sodium salicylate, then using sulfuric acid and produce crude product. The crude product through the sublimation refined to the finished product. Raw materials consumption quota: phenol (98%) 704kg/t, alkali burn (95%) 417kg/t, sulfate (95%) 500kg/t, carbon dioxide (99%) 467kg/t.
2.The preparation method of the method is that the sodium salt of phenol and carbon dioxide can be obtained by acidification.
phenol and liquid caustic soda are produced into solution of the sodium salt of phenol, vacuum drying, and then to 100℃, slowly put to the dry carbon dioxide, when the pressure reaches 0.7~0.8MPa, stop passing carbon dioxide, warming up to 140 to 180℃. After the reaction with water, sodium salicylate dissolved and decolorization, filtering, coupled with the sulfuric acid, namely precipitation salicylic acid, after filtering, washing and drying to obtain the product. ;
Well-known Reagent Company Product Information
Salicylic acid, 99+%(69-72-7)
Acros Organics
Salicylic acid, ACS, 99+%(69-72-7)
Alfa Aesar
69-72-7(sigmaaldrich)
Sigma Aldrich
2-Hydroxybenzoic Acid,>99.5%(T)(69-72-7)
TCI AMERICA
Supplier
Shanghai Kangtuo Chemical Co., Ltd.
Telephone021-69185552 13701777608
Websitehttp://www.kangtuochem.com
Shanghai Aladdin Bio-Chem Technology Co.,LTD
Telephone400-400-6206333 18521732826
Websitehttp://www.aladdin-e.com/
Hefei TNJ Chemical Industry Co.,Ltd.
Telephone551-65418679 15837135945
Websitehttps://www.tnjchem.com
Shandong Xuchen Chemical Technology Co. Ltd
Telephone0533-13853380-763 13853380763
Websitehttps://www.chemicalbook.com/supplier/15604011/1.htm
Langfang Ganyao Technology Co., LTD
Telephone0316-2617888 17301291189
Websitehttp://www.pc-chem.com/
Spec-Chem Industry Inc.
Telephone 13505162978
Websitehttp://www.specchemind.com
SHANDONGQIANGSENHUAGONG
Telephone0533-18369966-222 18369966222
Websitehttps://www.chemicalbook.com/supplier/23325451/
Shandong Debek Bioengineering Co. LTD
Telephone13854830608; 13854830608
Website
ZIBO LISHUO CHEMICAL
Telephone 13853320569
Websitehttps://www.chemicalbook.com/supplier/25771343/
J & K SCIENTIFIC LTD.
Telephone010-82848833 400-666-7788
Websitehttp://www.jkchemical.com
Meryer (Shanghai) Chemical Technology Co., Ltd.
Telephone021-61259108 18621169109
Websitehttps://www.meryer.com/cn/index/
3B Pharmachem (Wuhan) International Co.,Ltd.
Telephone821-50328103-801 18930552037
Websitehttps://www.chemicalbook.com/ShowSupplierProductsList13285/0.htm
future industrial shanghai co., ltd
Telephone400-0066400 13621662912
Websitehttp://www.jonln.com
Alfa Aesar
Telephone400-6106006
Websitehttp://chemicals.thermofisher.cn
TCI (Shanghai) Development Co., Ltd.
Telephone021-67121386
Websitehttps://www.tcichemicals.com/CN/zh/
Beijing HwrkChemical Technology Co., Ltd
Telephone010-89508211 18501085097
Websitehttp://www.hwrkchemical.com
Beijing Ouhe Technology Co., Ltd
Telephone010-82967028 13552068683
Websitehttp://www.ouhechem.com/
JinYan Chemicals(ShangHai) Co.,Ltd.
Telephone13817811078
Websitehttp://www.jingyan-chemical.com/
1of4
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine